DITROPAN
XL- oxybutynin chloride tablet, extended release
Janssen Pharmaceuticals, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DITROPAN XL
® safely and effectively. See full prescribing information for DITROPAN XL
®.
DITROPAN XL ® (oxybutynin chloride) Extended Release Tablets for oral use Initial U.S. Approval: 1975 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATIONDITROPAN XL ® must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed. DITROPAN XL ® may be administered with or without food. ( 2) DOSAGE FORMS AND STRENGTHSExtended release tablets 5 mg and 10 mg ( 3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common (incidence ≥5%) adverse reactions were dry mouth, constipation, diarrhea, headache, somnolence, and dizziness. ( 6) To report SUSPECTED ADVERSE REACTIONS, contact Janssen Pharmaceuticals, Inc. at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2021 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
DITROPAN XL ® (oxybutynin chloride) is a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency.
DITROPAN XL ® is also indicated for the treatment of pediatric patients aged 6 years and older with symptoms of detrusor overactivity associated with a neurological condition (e.g., spina bifida).
2 DOSAGE AND ADMINISTRATION
DITROPAN XL ® must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed.
DITROPAN XL ® may be administered with or without food.
2.1 Adults
The recommended starting dose of DITROPAN XL ® is 5 or 10 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 30 mg/day). In general, dosage adjustment may proceed at approximately weekly intervals.
3 DOSAGE FORMS AND STRENGTHS
DITROPAN XL ® extended-release tablets are available as 5 and 10 mg tablets for oral use:
5 mg: Pale yellow, round, tablet with "5 XL" printed on one side with black ink.
10 mg: Pink, round, tablet with "10 XL" printed on one side with black ink.
4 CONTRAINDICATIONS
DITROPAN XL ® is contraindicated in patients with urinary retention, gastric retention and other severe decreased gastrointestinal motility conditions, uncontrolled narrow-angle glaucoma.
DITROPAN XL ® is also contraindicated in patients who have demonstrated hypersensitivity to the drug substance or other components of the product. There have been reports of hypersensitivity reactions, including anaphylaxis and angioedema.
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
Angioedema of the face, lips, tongue and/or larynx has been reported with oxybutynin. In some cases, angioedema occurred after the first dose. Angioedema associated with upper airway swelling may be life-threatening. If involvement of the tongue, hypopharynx, or larynx occurs, oxybutynin should be promptly discontinued and appropriate therapy and/or measures necessary to ensure a patent airway should be promptly provided.
5.2 Central Nervous System Effects
Oxybutynin is associated with anticholinergic central nervous system (CNS) effects [see Adverse Reactions (6)] . A variety of CNS anticholinergic effects have been reported, including hallucinations, agitation, confusion and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly in the first few months after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how DITROPAN XL ® affects them. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
DITROPAN XL ® should be used with caution in patients with preexisting dementia treated with cholinesterase inhibitors due to the risk of aggravation of symptoms.
DITROPAN XL ® should be used with caution in patients with Parkinson's disease due to the risk of aggravation of symptoms.
5.3 Worsening of Symptoms of Myasthenia Gravis
DITROPAN XL ® should be used with caution in patients with myasthenia gravis due to the risk of aggravation of symptoms.
5.4 Worsening of Symptoms of Decreased Gastrointestinal Motility in Patients with Autonomic Neuropathy
DITROPAN XL ® should be used with caution in patients with autonomic neuropathy due to the risk of aggravation of symptoms of decreased gastrointestinal motility.
5.5 Urinary Retention
DITROPAN XL ® should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention [see Contraindications (4)] .
5.6 Gastrointestinal Adverse Reactions
DITROPAN XL ® should be administered with caution to patients with gastrointestinal obstructive disorders because of the risk of gastric retention [see Contraindications (4)] .
DITROPAN XL ®, like other anticholinergic drugs, may decrease gastrointestinal motility and should be used with caution in patients with conditions such as ulcerative colitis and intestinal atony.
DITROPAN XL ® should be used with caution in patients who have gastroesophageal reflux and/or who are concurrently taking drugs (such as bisphosphonates) that can cause or exacerbate esophagitis.
As with any other nondeformable material, caution should be used when administering DITROPAN XL ® to patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic). There have been rare reports of obstructive symptoms in patients with known strictures in association with the ingestion of other drugs in nondeformable controlled-release formulations.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety and efficacy of DITROPAN XL ® (5 to 30 mg/day) was evaluated in 774 adult subjects who participated in five double-blind, controlled clinical trials. In four of the five studies, Ditropan IR (5 to 20 mg/day in 199 subjects) was an active comparator. Adverse reactions reported by ≥ 1% of subjects are shown in Table 1.
| System/Organ Class
Preferred Term | DITROPAN XL
®
5 to 30 mg/day n=774 % | Ditropan IR
*
5 to 20 mg/day n=199 % |
|---|---|---|
| Psychiatric Disorders | ||
| Insomnia | 3.0 | 5.5 |
| Nervous System Disorders | ||
| Headache | 7.5 | 8.0 |
| Somnolence | 5.6 | 14.1 |
| Dizziness | 5.0 | 16.6 |
| Dysgeusia | 1.6 | 1.5 |
| Eye Disorders | ||
| Vision blurred | 4.3 | 9.6 |
| Dry eye | 3.1 | 2.5 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough | 1.9 | 3.0 |
| Oropharyngeal pain | 1.9 | 1.5 |
| Dry throat | 1.7 | 2.5 |
| Nasal dryness | 1.7 | 4.5 |
| Gastrointestinal Disorders | ||
| Dry mouth | 34.9 | 72.4 |
| Constipation | 8.7 | 15.1 |
| Diarrhea | 7.9 | 6.5 |
| Dyspepsia | 4.5 | 6.0 |
| Nausea | 4.5 | 11.6 |
| Abdominal pain | 1.6 | 2.0 |
| Vomiting | 1.3 | 1.5 |
| Flatulence | 1.2 | 2.5 |
| Gastro-esophageal reflux disease | 1.0 | 0.5 |
| Skin and Subcutaneous Tissue Disorders | ||
| Dry skin | 1.8 | 2.5 |
| Pruritus | 1.3 | 1.5 |
| Renal and Urinary Disorders | ||
| Dysuria | 1.9 | 2.0 |
| Urinary hesitation | 1.9 | 8.5 |
| Urinary retention | 1.2 | 3.0 |
| General Disorders and Administration Site Conditions | ||
| Fatigue | 2.6 | 3.0 |
| Investigations | ||
| Residual urine volume † | 2.3 | 3.5 |
The discontinuation rate due to adverse reactions was 4.4% with DITROPAN XL ® compared to 0% with Ditropan IR. The most frequent adverse reaction causing discontinuation of study medication was dry mouth (0.7%).
The following adverse reactions were reported by <1% of DITROPAN XL ®-treated patients and at a higher incidence than placebo in clinical trials: Metabolism and Nutrition Disorders: anorexia, fluid retention; Vascular disorders: hot flush; Respiratory, thoracic and mediastinal disorders: dysphonia; Gastrointestinal Disorders: dysphagia, frequent bowel movements; General disorders and administration site conditions: chest discomfort, thirst.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported from worldwide postmarketing experience with DITROPAN XL ®. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections and Infestations: Urinary tract infection; Psychiatric Disorders: psychotic disorder, agitation, confusional state, hallucinations, memory impairment, abnormal behavior; Nervous System Disorders: convulsions; Eye Disorders: glaucoma; Respiratory, Thoracic and Mediastinal Disorders: nasal congestion; Cardiac Disorders: arrhythmia, tachycardia, palpitations, QT interval prolongation; Vascular Disorders: flushing, hypertension; Skin and Subcutaneous Tissue Disorders: rash; Renal and Urinary Disorders: impotence; General Disorders and Administration Site Conditions: hypersensitivity reactions, including angioedema with airway obstruction, urticaria, and face edema; anaphylactic reactions requiring hospitalization for emergency treatment; Injury, poisoning and procedural complications: fall.
Additional adverse events reported with some other oxybutynin chloride formulations include: cycloplegia, mydriasis, and suppression of lactation. In one reported case, concomitant use of oxybutynin with carbamazepine and dantrolene was associated with adverse events of vomiting, drowsiness, confusion, unsteadiness, slurred speech and nystagmus, suggestive of carbamazepine toxicity.
7 DRUG INTERACTIONS
The concomitant use of oxybutynin with other anticholinergic drugs or with other agents which produce dry mouth, constipation, somnolence (drowsiness), and/or other anticholinergic-like effects may increase the frequency and/or severity of such effects.
Anticholinergic agents may potentially alter the absorption of some concomitantly administered drugs due to anticholinergic effects on gastrointestinal motility. This may be of concern for drugs with a narrow therapeutic index. Anticholinergic agents may also antagonize the effects of prokinetic agents, such as metoclopramide.
Mean oxybutynin plasma concentrations were approximately 2 fold higher when DITROPAN XL ® was administered with ketoconazole, a potent CYP3A4 inhibitor. Other inhibitors of the cytochrome P450 3A4 enzyme system, such as antimycotic agents (e.g., itraconazole and miconazole) or macrolide antibiotics (e.g., erythromycin and clarithromycin), may alter oxybutynin mean pharmacokinetic parameters (i.e., C max and AUC). The clinical relevance of such potential interactions is not known. Caution should be used when such drugs are co-administered.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on DITROPAN XL ® use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
8.2 Lactation
Risk Summary
There are no data on the presence of oxybutynin in human milk, the effects on the breastfed infant, or the effects of DITROPAN XL ® on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DITROPAN XL ® and any potential adverse effects on the breastfed child from DITROPAN XL ® or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of DITROPAN XL ® were studied in 60 children in a 24-week, open-label, non-randomized trial. Patients were aged 6–15 years, all had symptoms of detrusor overactivity in association with a neurological condition (e.g., spina bifida), all used clean intermittent catheterization, and all were current users of oxybutynin chloride. Study results demonstrated that administration of DITROPAN XL ® 5 to 20 mg/day was associated with an increase from baseline in mean urine volume per catheterization from 108 mL to 136 mL, an increase from baseline in mean urine volume after morning awakening from 148 mL to 189 mL, and an increase from baseline in the mean percentage of catheterizations without a leaking episode from 34% to 51%.
Urodynamic results were consistent with clinical results. Administration of DITROPAN XL ® resulted in an increase from baseline in mean maximum cystometric capacity from 185 mL to 254 mL, a decrease from baseline in mean detrusor pressure at maximum cystometric capacity from 44 cm H 2O to 33 cm H 2O, and a reduction in the percentage of patients demonstrating uninhibited detrusor contractions (of at least 15 cm H 2O) from 60% to 28%.
The pharmacokinetics of DITROPAN XL ® in these patients were consistent with those reported for adults [see Clinical Pharmacology (12.3)] .
DITROPAN XL ® is not recommended in pediatric patients who cannot swallow the tablet whole without chewing, dividing, or crushing, or in children under the age of 6.
8.5 Geriatric Use
The rate and severity of anticholinergic effects reported by patients less than 65 years old and those 65 years and older were similar. The pharmacokinetics of DITROPAN XL ® were similar in all patients studied (up to 78 years of age).
10 OVERDOSAGE
The continuous release of oxybutynin from DITROPAN XL ® should be considered in the treatment of overdosage. Patients should be monitored for at least 24 hours. Treatment should be symptomatic and supportive. A cathartic may be administered.
Overdosage with oxybutynin chloride has been associated with anticholinergic effects including central nervous system excitation, flushing, fever, dehydration, cardiac arrhythmia, vomiting, and urinary retention.
Ingestion of 100 mg oxybutynin chloride in association with alcohol has been reported in a 13-year-old boy who experienced memory loss, and a 34-year-old woman who developed stupor, followed by disorientation and agitation on awakening, dilated pupils, dry skin, cardiac arrhythmia, and retention of urine. Both patients fully recovered with symptomatic treatment.
11 DESCRIPTION
DITROPAN XL ® (oxybutynin chloride) is an antispasmodic, muscarinic antagonist. Each DITROPAN XL ® extended-release tablet contains 5 mg or 10 mg of oxybutynin chloride USP, formulated as a once-a-day controlled-release tablet for oral administration. Oxybutynin chloride is administered as a racemate of R- and S-enantiomers.
Chemically, oxybutynin chloride is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate hydrochloride. The empirical formula of oxybutynin chloride is C 22H 31NO 3•HCl.
Its structural formula is:
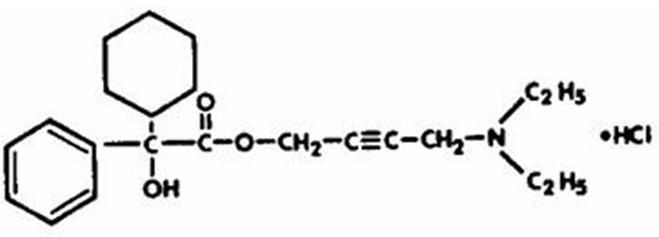
Oxybutynin chloride is a white crystalline solid with a molecular weight of 393.9. It is readily soluble in water and acids, but relatively insoluble in alkalis.
DITROPAN XL ® also contains the following inert ingredients: butylated hydroxytoluene, cellulose acetate, hypromellose, lactose, magnesium stearate, polyethylene glycol, polyethylene oxide, polysorbate 80, propylene glycol, sodium chloride, synthetic iron oxides and titanium dioxide.
System Components and Performance
DITROPAN XL ® uses osmotic pressure to deliver oxybutynin chloride at a controlled rate over approximately 24 hours. The system, which resembles a conventional tablet in appearance, comprises an osmotically active bilayer core surrounded by a semipermeable membrane. The bilayer core is composed of a drug layer containing the drug and excipients, and a push layer containing osmotically active components. There is a precision-laser drilled orifice in the semipermeable membrane on the drug-layer side of the tablet. In an aqueous environment, such as the gastrointestinal tract, water permeates through the membrane into the tablet core, causing the drug to go into suspension and the push layer to expand. This expansion pushes the suspended drug out through the orifice. The semipermeable membrane controls the rate at which water permeates into the tablet core, which in turn controls the rate of drug delivery. The controlled rate of drug delivery into the gastrointestinal lumen is thus independent of pH or gastrointestinal motility. The function of DITROPAN XL ® depends on the existence of an osmotic gradient between the contents of the bilayer core and the fluid in the gastrointestinal tract. Since the osmotic gradient remains constant, drug delivery remains essentially constant. The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the feces as an insoluble shell.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Oxybutynin relaxes bladder smooth muscle. Oxybutynin chloride exerts a direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine on smooth muscle. No blocking effects occur at skeletal neuromuscular junctions or autonomic ganglia (antinicotinic effects).
Antimuscarinic activity resides predominantly in the R-isomer. A metabolite, desethyloxybutynin, has pharmacological activity similar to that of oxybutynin in in vitro studies.
12.2 Pharmacodynamics
In patients with conditions characterized by involuntary bladder contractions, cystometric studies have demonstrated that oxybutynin increases bladder (vesical) capacity, diminishes the frequency of uninhibited contractions of the detrusor muscle, and delays the initial desire to void.
12.3 Pharmacokinetics
Absorption
Following the first dose of DITROPAN XL ®, oxybutynin plasma concentrations rise for 4 to 6 hours; thereafter steady concentrations are maintained for up to 24 hours, minimizing fluctuations between peak and trough concentrations associated with oxybutynin.
The relative bioavailabilities of R- and S-oxybutynin from DITROPAN XL ® are 156% and 187%, respectively, compared with oxybutynin. The mean pharmacokinetic parameters for R- and S-oxybutynin are summarized in Table 2. The plasma concentration-time profiles for R- and S-oxybutynin are similar in shape; Figure 1 shows the profile for R-oxybutynin.
| Parameters (units) | R-Oxybutynin | S-Oxybutynin | ||
|---|---|---|---|---|
| C max (ng/mL) | 1.0 | (0.6) | 1.8 | (1.0) |
| T max (h) | 12.7 | (5.4) | 11.8 | (5.3) |
| t 1/2 (h) | 13.2 | (6.2) | 12.4 | (6.1) |
| AUC (0–48) (ng∙h/mL) | 18.4 | (10.3) | 34.2 | (16.9) |
| AUC inf (ng∙h/mL) | 21.3 | (12.2) | 39.5 | (21.2) |
Figure 1: Mean R-oxybutynin plasma concentrations following a single dose of DITROPAN XL ® 10 mg and oxybutynin 5 mg administered every 8 hours (n=23 for each treatment).
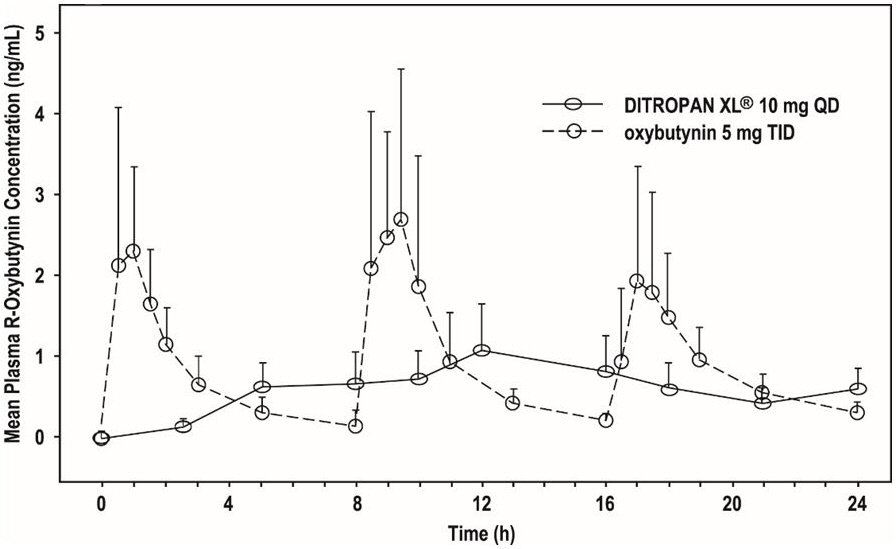
Steady state oxybutynin plasma concentrations are achieved by Day 3 of repeated DITROPAN XL ® dosing, with no observed drug accumulation or change in oxybutynin and desethyloxybutynin pharmacokinetic parameters.
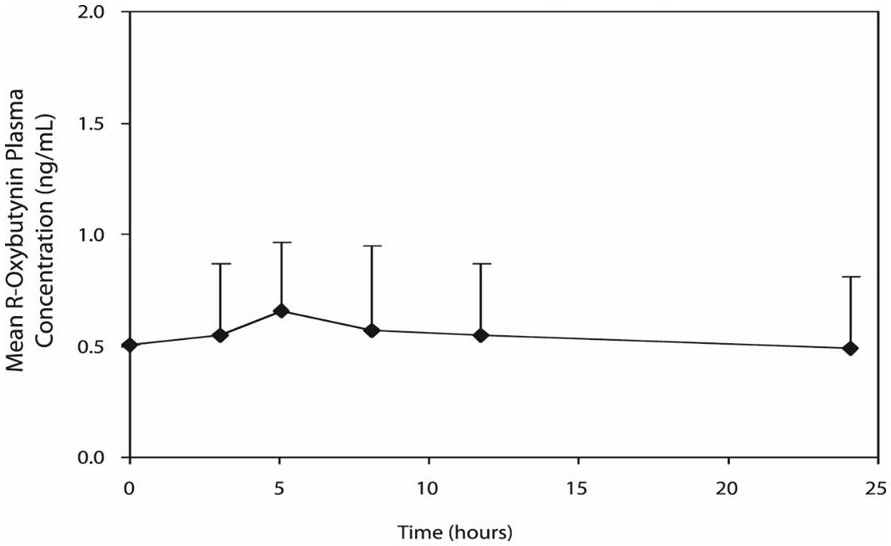
DITROPAN XL ® steady state pharmacokinetics were studied in 19 children aged 5–15 years with detrusor overactivity associated with a neurological condition (e.g., spina bifida). The children were on DITROPAN XL ® total daily dose ranging from 5 to 20 mg (0.10 to 0.77 mg/kg). Sparse sampling technique was used to obtain serum samples. When all available data are normalized to an equivalent of 5 mg per day of DITROPAN XL ®, the mean pharmacokinetic parameters derived for R- and S-oxybutynin and R- and S-desethyloxybutynin are summarized in Table 3. The plasma-time concentration profiles for R- and S-oxybutynin are similar in shape; Figure 2 shows the profile for R-oxybutynin when all available data are normalized to an equivalent of 5 mg per day.
| R-Oxybutynin | S-Oxybutynin | R- Desethyloxybutynin | S- Desethyloxybutynin | |
|---|---|---|---|---|
| C max (ng/mL) | 0.7 ± 0.4 | 1.3 ± 0.8 | 7.8 ± 3.7 | 4.2 ± 2.3 |
| T max (h) | 5.0 | 5.0 | 5.0 | 5.0 |
| AUC (ng∙h/mL) | 12.8 ± 7.0 | 23.7 ± 14.4 | 125.1 ± 66.7 | 73.6 ± 47.7 |
Figure 2: Mean steady state (± SD) R-oxybutynin plasma concentrations following administration of 5 to 20 mg DITROPAN XL ® once daily in children aged 5–15. Plot represents all available data normalized to an equivalent of DITROPAN XL ® 5 mg once daily.
Distribution
Oxybutynin is widely distributed in body tissues following systemic absorption. The volume of distribution is 193 L after intravenous administration of 5 mg oxybutynin chloride. Both enantiomers of oxybutynin are highly bound (>99%) to plasma proteins. Both enantiomers of N-desethyloxybutynin are also highly bound (>97%) to plasma proteins. The major binding protein is alpha-1 acid glycoprotein.
Metabolism
Oxybutynin is metabolized primarily by the cytochrome P450 enzyme systems, particularly CYP3A4 found mostly in the liver and gut wall. Its metabolic products include phenylcyclohexylglycolic acid, which is pharmacologically inactive, and desethyloxybutynin, which is pharmacologically active. Following DITROPAN XL ® administration, plasma concentrations of R- and S-desethyloxybutynin are 73% and 92%, respectively, of concentrations observed with oxybutynin.
Excretion
Oxybutynin is extensively metabolized by the liver, with less than 0.1% of the administered dose excreted unchanged in the urine. Also, less than 0.1% of the administered dose is excreted as the metabolite desethyloxybutynin.
Dose Proportionality
Pharmacokinetic parameters of oxybutynin and desethyloxybutynin (C max and AUC) following administration of 5–20 mg of DITROPAN XL ® are dose proportional.
Use in Specific Populations
Pediatric
The pharmacokinetics of DITROPAN XL ® were evaluated in 19 children aged 5–15 years with detrusor overactivity associated with a neurological condition (e.g., spina bifida). The pharmacokinetics of DITROPAN XL ® in these pediatric patients were consistent with those reported for adults (see Tables 2 and 3, and Figures 1 and 2 above).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A 24-month study in rats at dosages of oxybutynin chloride of 20, 80, and 160 mg/kg/day showed no evidence of carcinogenicity. These doses are approximately 6, 25, and 50 times the maximum human exposure, based on a human equivalent dose taking into account normalization of body surface area.
14 CLINICAL STUDIES
DITROPAN XL ® was evaluated for the treatment of patients with overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in three controlled efficacy studies. The majority of patients were Caucasian (89.0%) and female (91.9%) with a mean age of 59 years (range, 18 to 98 years). Entry criteria required that patients have urge or mixed incontinence (with a predominance of urge) as evidenced by ≥ 6 urge incontinence episodes per week and ≥ 10 micturitions per day. Study 1 was a fixed-dose escalation design, whereas the other two studies used a dose-adjustment design in which each patient's final dose was adjusted to a balance between improvement of incontinence symptoms and tolerability of side effects. All three studies included patients known to be responsive to oxybutynin or other anticholinergic medications, and these patients were maintained on a final dose for up to 2 weeks.
The efficacy results for the three controlled trials are presented in the following Tables 4, 5, and 6 and Figures 3, 4, and 5.
| Study 1 | n | DITROPAN XL ® | n | Placebo |
|---|---|---|---|---|
| Mean Baseline | 34 | 15.9 | 16 | 20.9 |
| Mean (SD) Change from Baseline * | 34 | -15.8 (8.9) | 16 | -7.6 (8.6) |
| 95% Confidence Interval for Difference | (-13.6, -2.8) † | |||
| (DITROPAN XL ® - Placebo) | ||||
Figure 3: Mean Change (±SD) in Urge Urinary Incontinence Episodes Per Week from Baseline (Study 1)
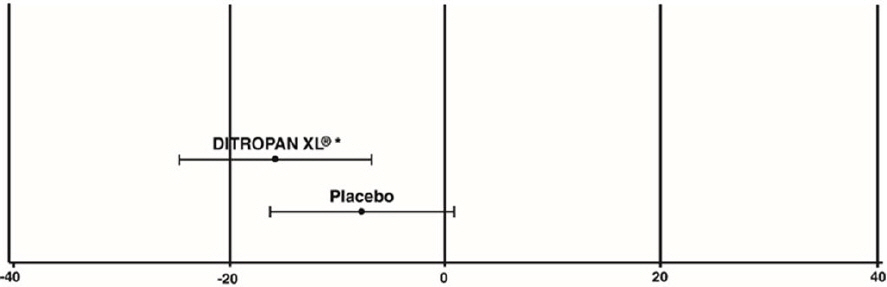
* The difference between DITROPAN XL ® and placebo was statistically significant.
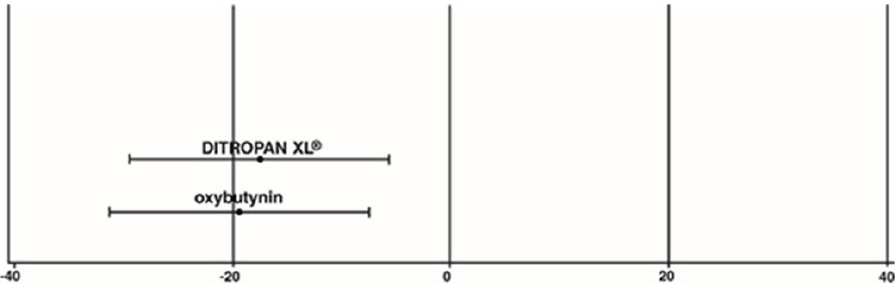
| Study 2 | n | DITROPAN XL ® | n | oxybutynin |
|---|---|---|---|---|
|
||||
| Mean Baseline | 53 | 27.6 | 52 | 23.0 |
| Mean (SD) Change from Baseline * | 53 | -17.6 (11.9) | 52 | -19.4 (11.9) |
| 95% Confidence Interval for Difference | (-2.8, 6.5) | |||
| (DITROPAN XL ® - oxybutynin) | ||||
Figure 4: Mean Change (±SD) in Urge Urinary Incontinence Episodes Per Week from Baseline (Study 2)
| Study 3 | n | DITROPAN XL ® | n | oxybutynin |
|---|---|---|---|---|
| Mean Baseline | 111 | 18.9 | 115 | 19.5 |
| Mean (SD) Change from Baseline * | 111 | -14.5 (8.7) | 115 | -13.8 (8.6) |
| 95% Confidence Interval for Difference | (-3.0, 1.6) † | |||
| (DITROPAN XL ® - oxybutynin) | ||||
Figure 5: Mean Change (±SD) in Urge Urinary Incontinence Episodes Per Week from Baseline (Study 3)
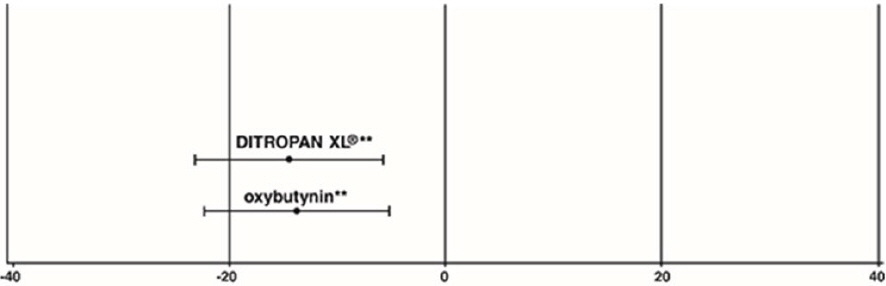
** The difference between DITROPAN XL ® and oxybutynin fulfilled the criteria for comparable efficacy.
16 HOW SUPPLIED/STORAGE AND HANDLING
DITROPAN XL ® extended-release tablets are available in two dosage strengths, 5 mg (pale yellow) and 10 mg (pink) and are imprinted on one side with "5 XL" or "10 XL" with black ink. DITROPAN XL ® extended-release tablets are supplied in bottles of 100 tablets.
| 5 mg | 100 count bottle | NDC 50458-805-01 |
| 10 mg | 100 count bottle | NDC 50458-810-01 |
17 PATIENT COUNSELING INFORMATION
- Patients should be informed that oxybutynin may produce angioedema that could result in life threatening airway obstruction. Patients should be advised to promptly discontinue oxybutynin therapy and seek immediate medical attention if they experience swelling of the tongue, edema of the laryngopharynx, or difficulty breathing.
- Patients should be informed that anticholinergic (antimuscarinic) agents such as DITROPAN XL
®, may produce clinically significant adverse reactions related to anticholinergic activity such as:
- Urinary retention and constipation
- Heat prostration due to decreased sweating. Heat prostration can occur when anticholinergic medicines are administered in the presence of high environmental temperature.
- Patients should be informed that anticholinergic medicines such as DITROPAN XL ® may produce drowsiness (somnolence), dizziness or blurred vision. Patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until DITROPAN XL ® effects have been determined.
- Patients should be informed that alcohol may enhance the drowsiness caused by anticholinergic agents such as DITROPAN XL ®.
- Patients should be informed that DITROPAN XL ® should be swallowed whole with the aid of liquids. Patients should not chew, divide, or crush tablets. The medication is contained within a nonabsorbable shell designed to release the drug at a controlled rate. The tablet shell is eliminated from the body; patients should not be concerned if they occasionally notice in their stool something that looks like a tablet.
- DITROPAN XL ® should be taken at approximately the same time each day.
For more information call 1-800-JANSSEN (1-800-526-7736).
Product of France
Manufactured by:
ALZA Corporation, Vacaville, CA 95688
An ALZA OROS
® Technology Product
DITROPAN XL ® and OROS ® are registered trademarks of ALZA Corporation.
Manufactured for:
Janssen Pharmaceuticals, Inc.
Titusville, NJ 08560
© 1998 Janssen Pharmaceutical Companies
| DITROPAN
XL
oxybutynin chloride tablet, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| DITROPAN
XL
oxybutynin chloride tablet, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Janssen Pharmaceuticals, Inc. (063137772) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PCAS France | 396133998 | api manufacture(50458-805, 50458-810) | |

