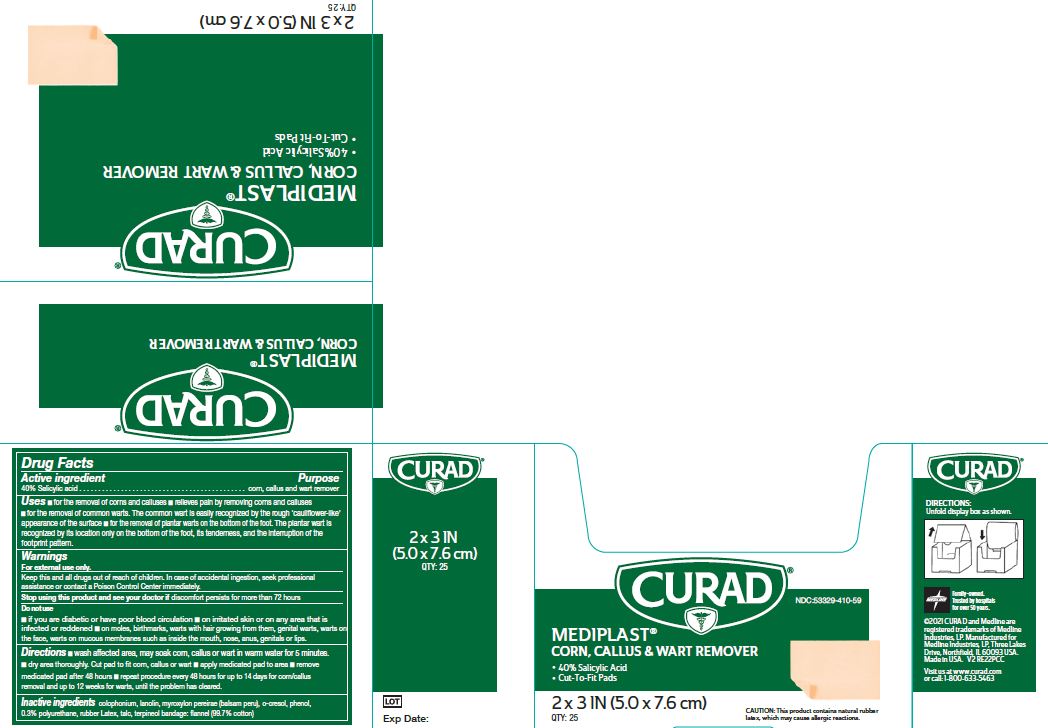
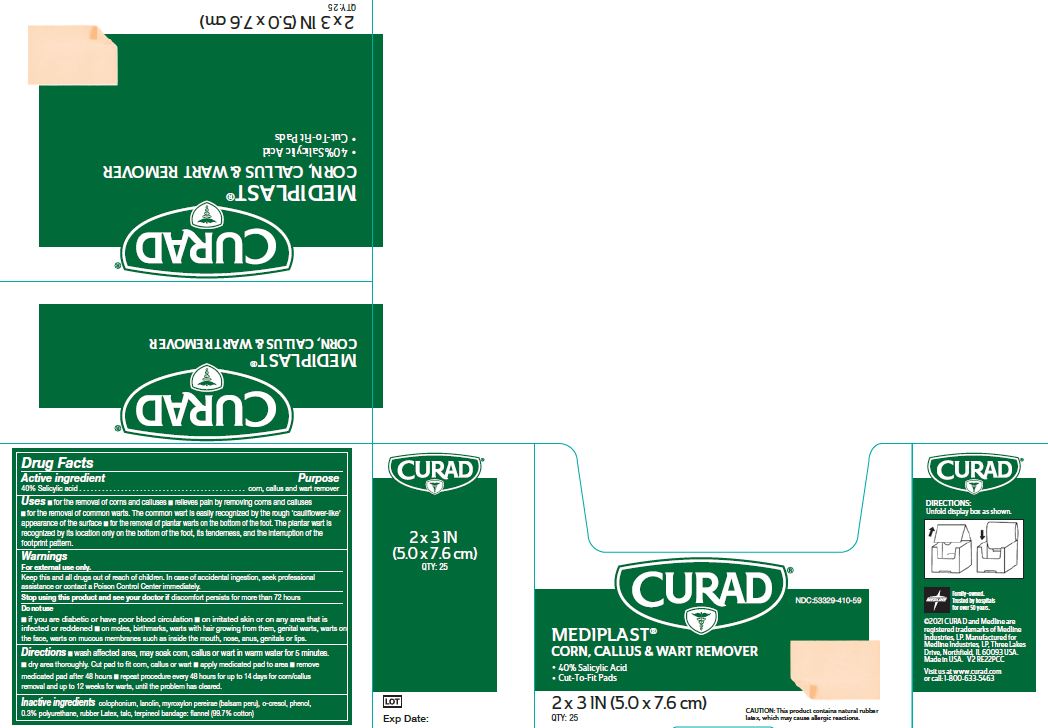
Label: MEDIPLAST- salicylic acid plaster
- NDC Code(s): 53329-410-09, 53329-410-59
- Packager: Medline Industries, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
-
Uses
- for the removal of corns and calluses
- Relieves pain by removing corns and calluses
- for the removal of common warts. The common wart is easily recognized by the rough ‘cauliflower-like’ appearance of the surface
- for the removal of plantar warts on the bottom of the foot. The plantar wart is recognized by its location only on the bottom of the foot, its tenderness, and the interruption of the footprint pattern.
-
Warnings
For external use only. This product contains natural rubber, which may cause allergic reactions.
Keep this and all drugs out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control center immediately.
Do Not Use
- if you are diabetic or have poor blood circulation
- on irritated skin or on any area that is infected or reddened
- on moles, birthmarks, or warts with hair growing from them, genital warts, warts on the face, warts on mucous membranes such as warts inside the mouth, nose, anus, genitals or lips.
-
Directions
- Wash affected area. May soak corn, callus or wart in warm water for 5 minutes. Dry area thoroughly. Cut pad to fit corn, callus or wart. Apply medicated pad to area. Remove medicated pad after 48 hours. Repeat procedure every 48 hours for up to 14 days for corn/callus removal and up to 12 weeks for warts, until the problem has cleared.
- Inactive ingredients
- Manufacturing Information
- Package Labels
-
INGREDIENTS AND APPEARANCE
MEDIPLAST
salicylic acid plasterProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53329-410 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 g in 100 g Inactive Ingredients Ingredient Name Strength PHENOL (UNII: 339NCG44TV) LANOLIN (UNII: 7EV65EAW6H) NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) BALSAM PERU (UNII: 8P5F881OCY) ROSIN (UNII: 88S87KL877) TALC (UNII: 7SEV7J4R1U) TERPINEOL (UNII: R53Q4ZWC99) ORTHOCRESOL (UNII: YW84DH5I7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53329-410-59 25 in 1 BOX 08/01/2008 1 1 in 1 PACKET 1 1.4 g in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:53329-410-09 1 in 1 PACKET 08/01/2008 2 1.4 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358F 08/01/2008 Labeler - Medline Industries, LP (025460908)