Label: BENZTROPINE MESYLATE injection
-
Contains inactivated NDC Code(s)
NDC Code(s): 10858-011-01, 10858-011-05 - Packager: Wintac Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 26, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
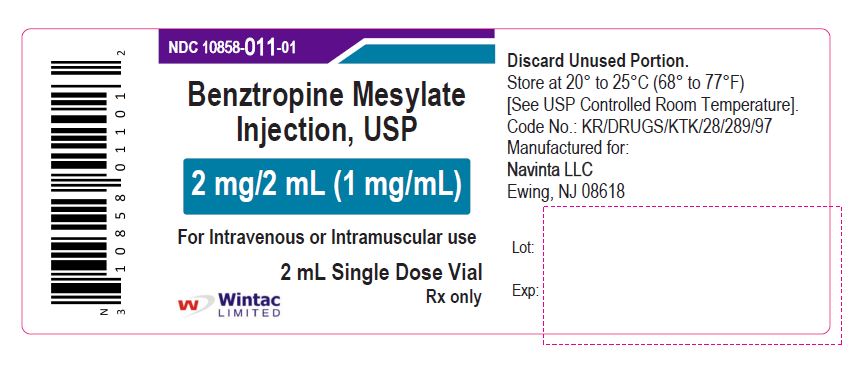
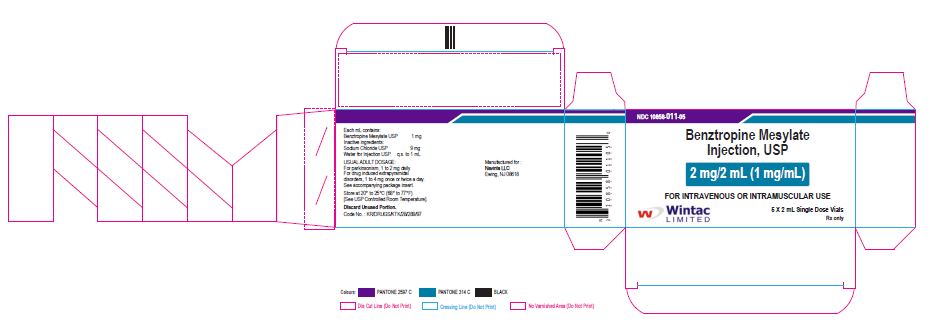
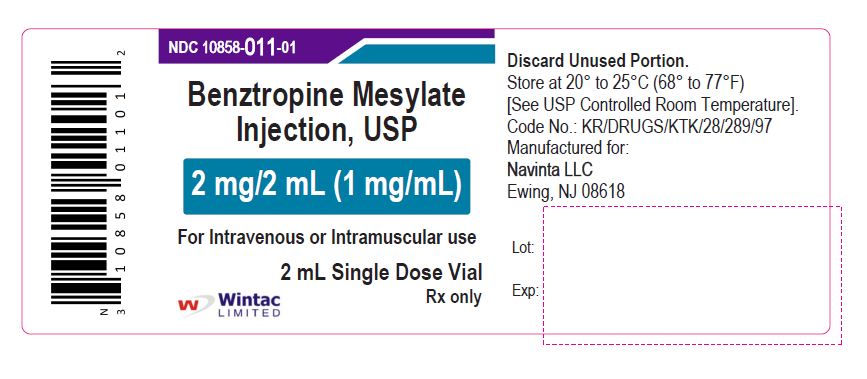
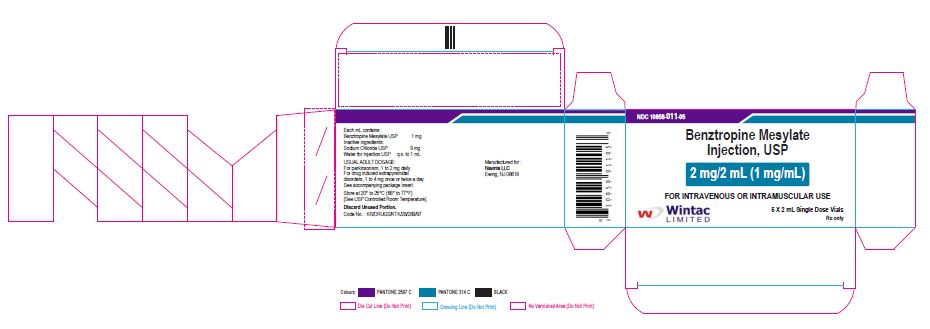
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENZTROPINE MESYLATE
benztropine mesylate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10858-011 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZTROPINE MESYLATE (UNII: WMJ8TL7510) (BENZTROPINE - UNII:1NHL2J4X8K) BENZTROPINE MESYLATE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10858-011-05 5 in 1 CARTON 11/26/2020 1 NDC:10858-011-01 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091525 11/26/2020 Labeler - Wintac Limited (677236695) Registrant - Navinta LLC (130443810)