ARIPIPRAZOLE - aripiprazole tablet, orally disintegrating
Cadila Healthcare Limited
----------
ARIPIPRAZOLE ORALLY DISINTEGRATING TABLETS
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
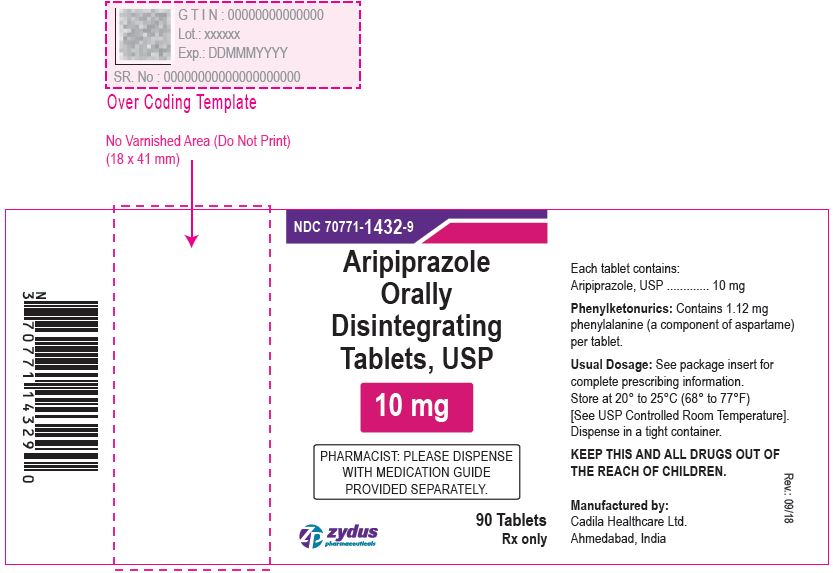
Aripiprazole Orally Disintegrating Tablets, 10 mg
90 tablets
Rx only
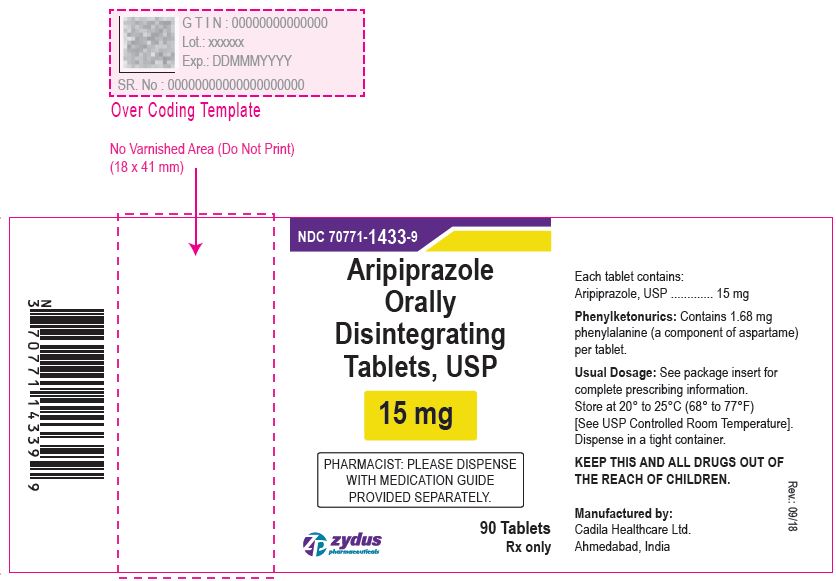
Aripiprazole Orally Disintegrating Tablets, 15 mg
90 tablets
Rx only
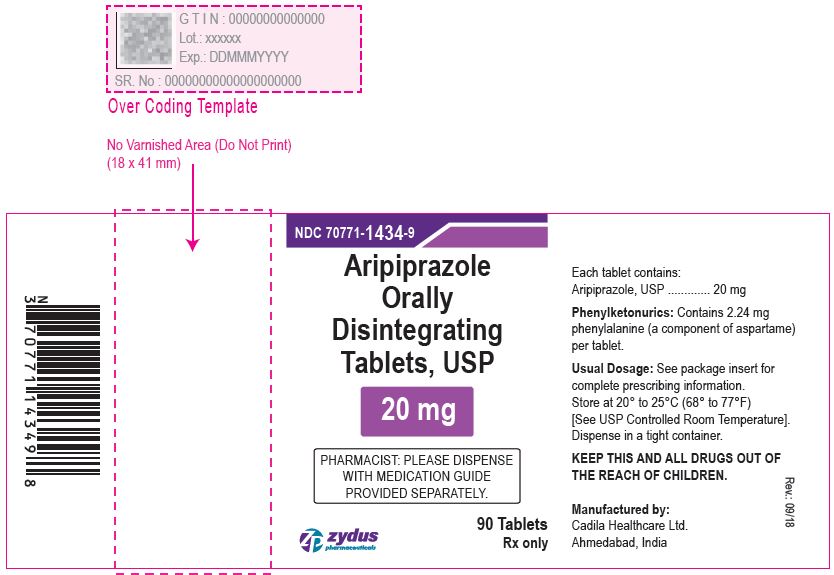
Aripiprazole Orally Disintegrating Tablets, 20 mg
90 tablets
Rx only
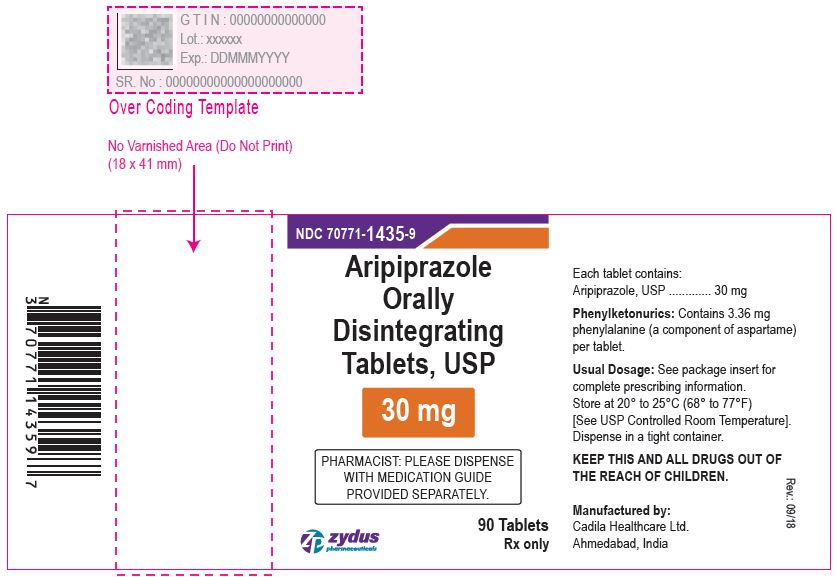
Aripiprazole Orally Disintegrating Tablets, 30 mg
90 tablets
Rx only
| ARIPIPRAZOLE
aripiprazole tablet, orally disintegrating |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ARIPIPRAZOLE
aripiprazole tablet, orally disintegrating |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ARIPIPRAZOLE
aripiprazole tablet, orally disintegrating |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ARIPIPRAZOLE
aripiprazole tablet, orally disintegrating |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Cadila Healthcare Limited (918596198) |
| Registrant - Cadila Healthcare Limited (918596198) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cadila Healthcare Limited | 918596198 | ANALYSIS(70771-1432, 70771-1433, 70771-1434, 70771-1435) , MANUFACTURE(70771-1432, 70771-1433, 70771-1434, 70771-1435) | |
Revised: 12/2021
Document Id: 87917ed0-9aa4-444a-b3bf-6fb4a829d268
Set id: 34d09d2e-5780-48bd-a6ea-046108ba84be
Version: 5
Effective Time: 20211230
Cadila Healthcare Limited