ORIBE SERENE SCALP ANTI DANDRUFF- oribe serene scalp anti dandruff lotion
Oribe Hair Care LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
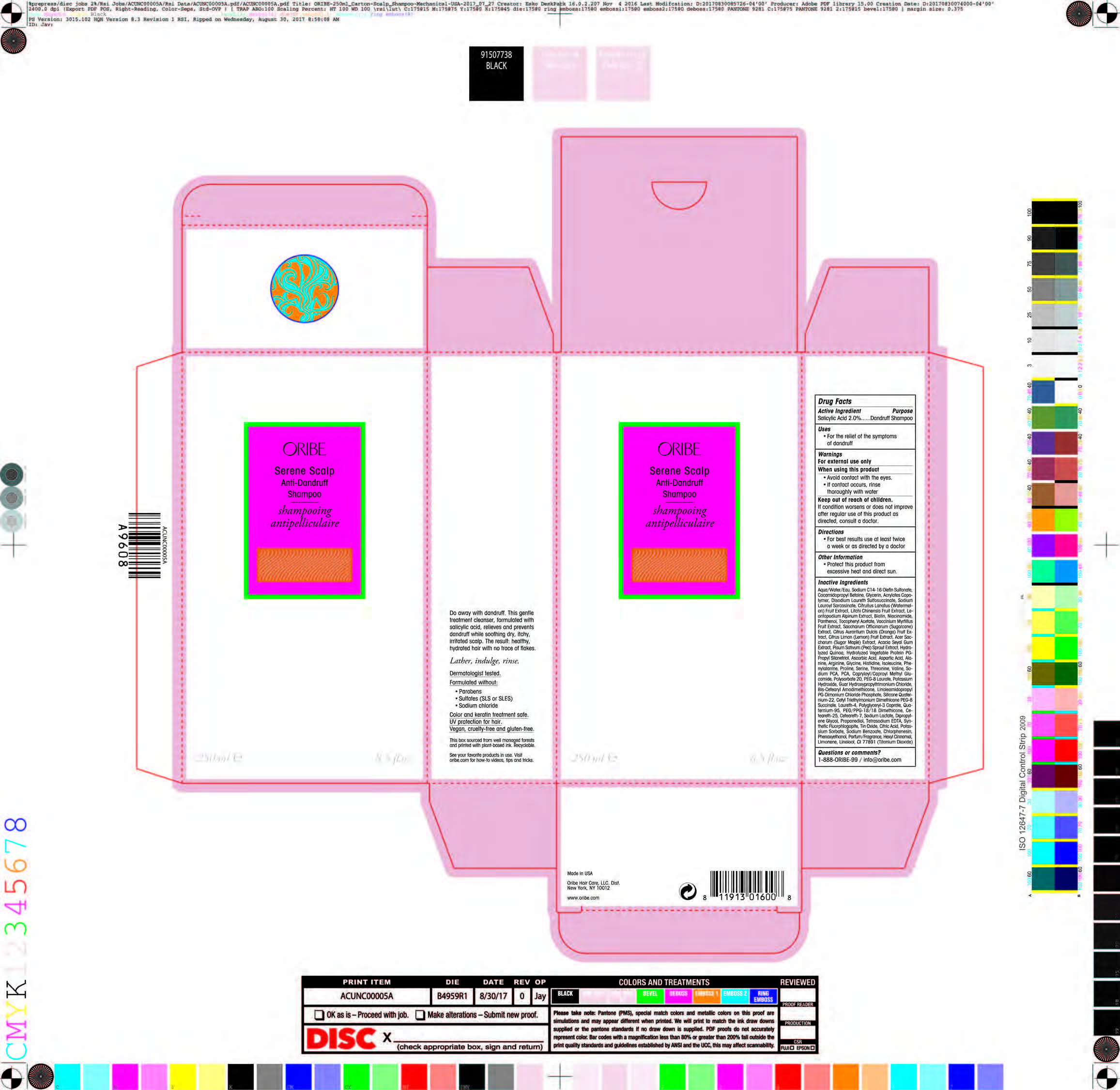
DRUG FACTS
Warnings
For external use only
When using this product
• Avoid contact wilh lhe eyes.
• lf conlacl occurs, rinse thoroughly wlth water
Keep out of reach of children.
Keep out of reach of children.
lf condition worsens or does nol improve alter regular use of this product as directed, consult a doctor.
Inactive Ingredients
Aqua/Water/Eau, Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Glycerin, Acrylates Copolymer, Disodium Laureth Sulfosuccinate, Sodium Lauroyl Sarcosinate, Citrullus Lanatus (Watermelon) Fruit Extract, Litchi Chinensis Fruit Extract, Leontopodium Alpinum Extract, Biotin, Niacinamide, Panthenol, Tocopheryl Acetate, Vaccinium Myrtillus Fruit Extract, Saccharum Officinarum (Sugarcane) Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Citrus Limon (Lemon) Fruit Extract, Acer Saccharum (Sugar Maple) Extract, Acacia Seyal Gum Extract, Pisum Sativum (Pea) Sprout Extract, Hydrolyzed Quinoa, Hydrolyzed Vegetable Protein PG-Propyl Silanetriol, Ascorbic Acid, Aspartic Acid, Alanine, Arginine, Glycine, Histidine, Isoleucine, Phenylalanine, Proline, Serine, Threonine, Valine, Sodium PCA, PCA, Capryloyl/Caproyl Methyl Glucamide, Polysorbate 20, PEG-8 Laurate, Potassium Hydroxide, Guar Hydroxypropyltrimonium Chloride, Bis-Cetearyl Amodimethicone, Linoleamidopropyl PG-Dimonium Chloride Phosphate, Silicone Quaternium-22, Cetyl Triethylmonium Dimethicone PEG-8 Succinate, Laureth-4, Polyglyceryl-3 Caprate, Quaternium-95, PEG/PPG-18/18 Dimethicone, Ceteareth-25, Ceteareth-7, Sodium Lactate, Dipropylene Glycol, Propanediol, Tetrasodium EDTA, Synthetic Fluorphlogopite, Tin Oxide, Citric Acid, Potassium Sorbate, Sodium Benzoate, Chlorphenesin, Phenoxyethanol, Parfum/Fragrance, Hexyl Cinnamal, Limonene, Linalool, CI 77891 (Titanium Dioxide)
| ORIBE SERENE SCALP ANTI DANDRUFF
oribe serene scalp anti dandruff lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Oribe Hair Care LLC (008843347) |
| Registrant - Oribe Hair Care LLC (008843347) |