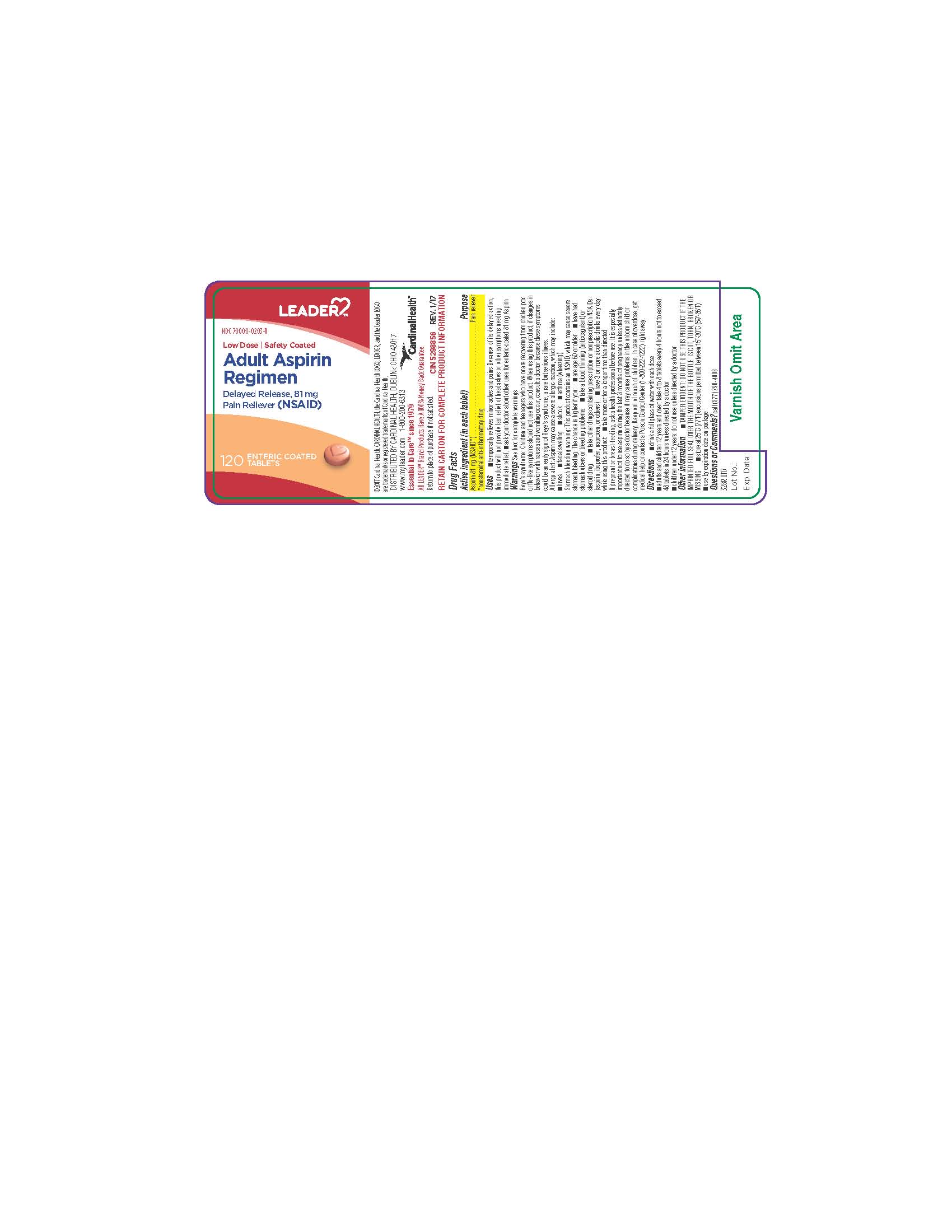
LOW DOSE ASPIRIN ENTERIC SAFETY COATED- aspirin tablet, delayed release
CARDINAL HEALTH
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LEADER 328R
|
Uses
|
| Anhydrous lactose, carnauba wax, colloidal silicon dioxide, croscamellose sodium, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, iron oxide ochre, methacrylic acid, ethyl acrylate copolymer, microcrystalline cellulose, polysorbate 80, simethicone, sodium hydroxide, sodium lauryl sulfate, talc, triethyl citrate |
|
WARNINGS: Reye’s Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s Syndrome, a rare but serious illness. Allergy Alert: Aspirin may cause a severe allergic reaction, which may include hives, facial swelling, shock, asthma (wheezing) Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you are: age 60 or older; have had stomach ulcers or bleeding problems; take a blood thinning (anticoagulant) or steroid drug; take more or for a longer time than directed. |
| Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800 222-1222) right away. |
| LOW DOSE ASPIRIN
ENTERIC SAFETY COATED
aspirin tablet, delayed release |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - CARDINAL HEALTH (097537435) |
| Registrant - TIME CAP LABORATORIES, INC (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TIME CAP LABORATORIES, INC | 037052099 | manufacture(70000-0203) | |