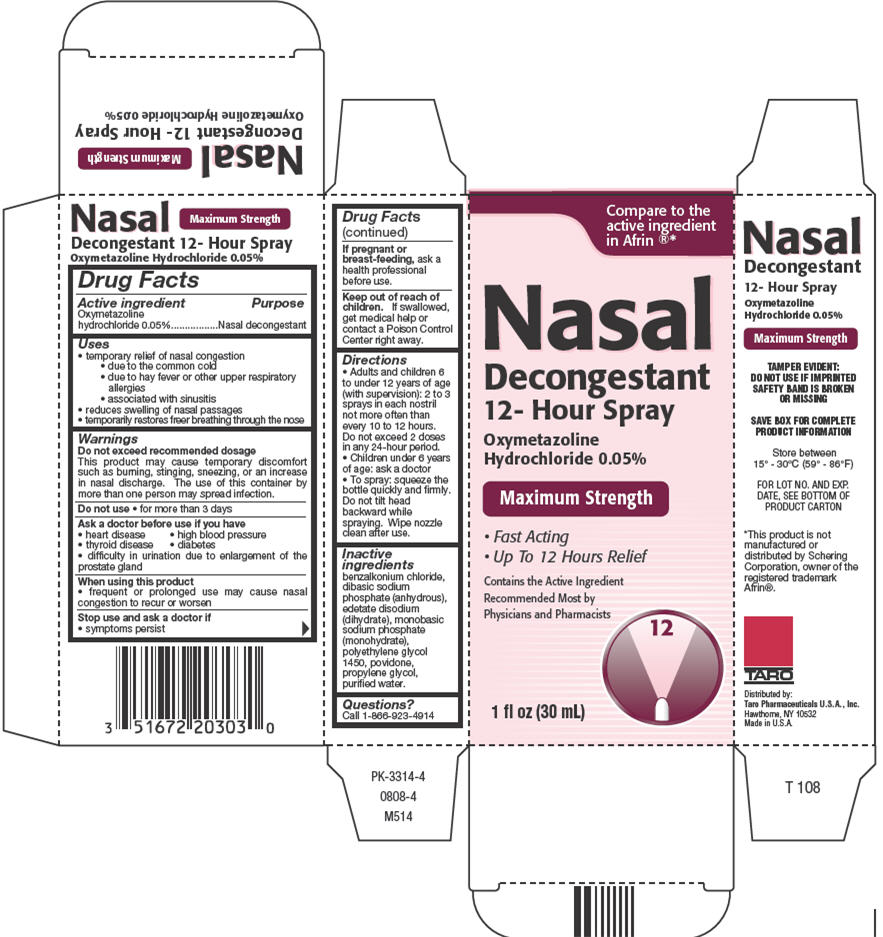
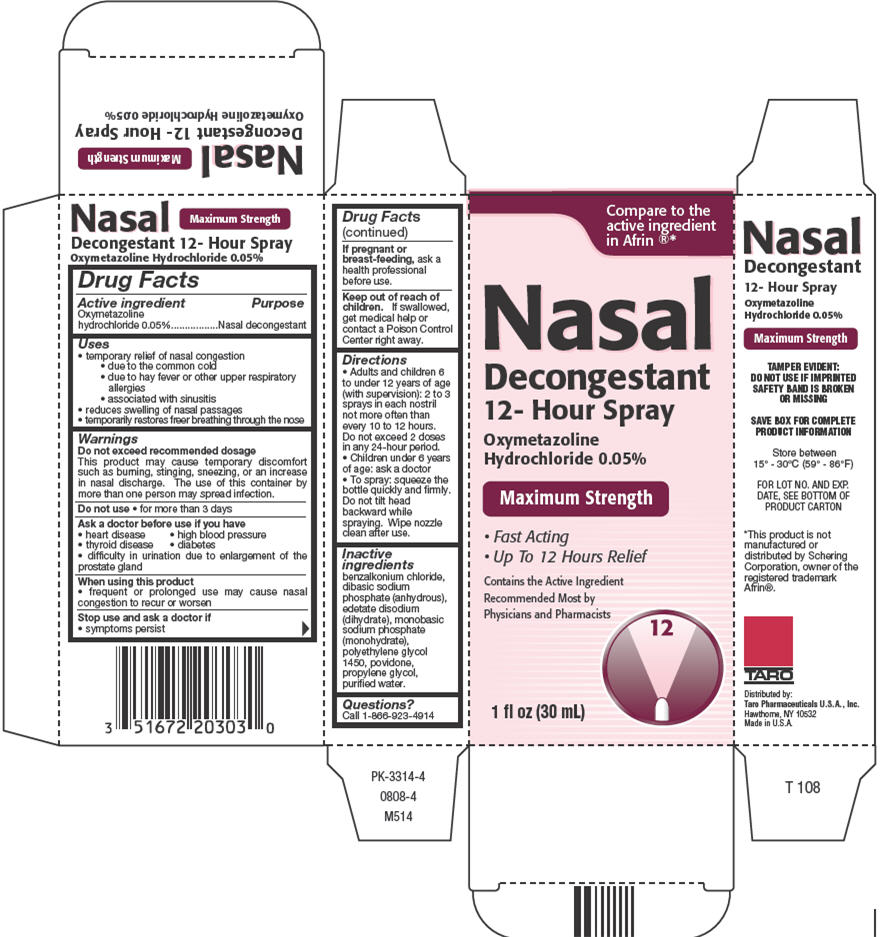
Label: OXYMETAZOLINE HYDROCHLORIDE 12-HOUR- oxymetazoline hydrochloride spray
- NDC Code(s): 51672-2030-3, 51672-2030-5
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 15, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Do not exceed recommended dosage
This product may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge. The use of this container by more than one person may spread infection.
-
Directions
- Adults and children 6 to under 12 years of age (with supervision): 2 to 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- Children under 6 years of age: ask a doctor
- To spray: squeeze the bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use.
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
OXYMETAZOLINE HYDROCHLORIDE 12-HOUR
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51672-2030 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Oxymetazoline Hydrochloride (UNII: K89MJ0S5VY) (Oxymetazoline - UNII:8VLN5B44ZY) Oxymetazoline Hydrochloride 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) sodium phosphate, dibasic, anhydrous (UNII: 22ADO53M6F) edetate disodium (UNII: 7FLD91C86K) sodium phosphate, monobasic, monohydrate (UNII: 593YOG76RN) polyethylene glycol 1450 (UNII: OJ4Z5Z32L4) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51672-2030-5 1 in 1 CARTON 02/25/2006 1 15 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC:51672-2030-3 1 in 1 CARTON 02/25/2006 2 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 02/25/2006 Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Applied Laboratories, Inc. 117337220 MANUFACTURE(51672-2030)