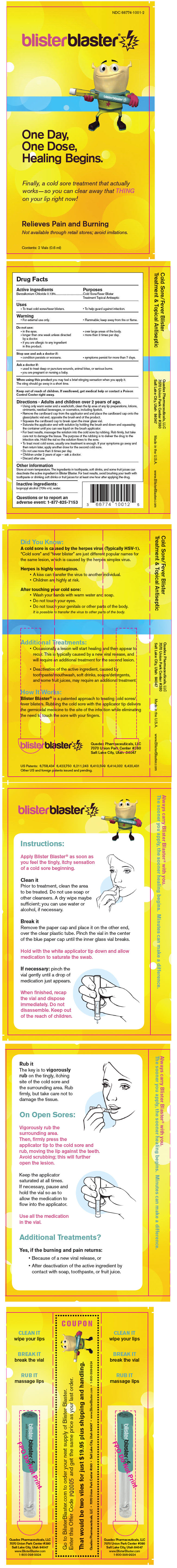
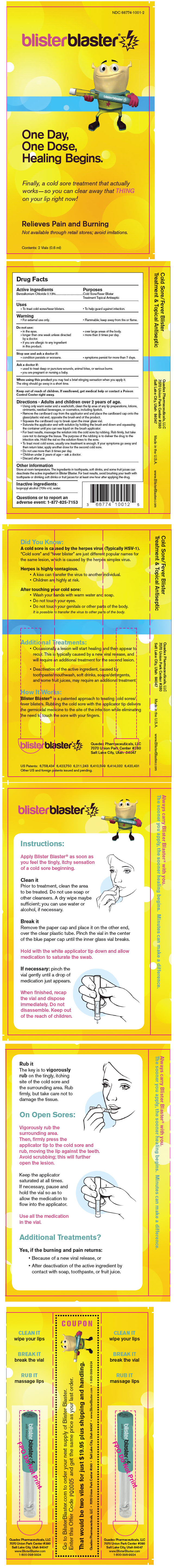
Label: BLISTER BLASTER- benzalkonium chloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 66774-1001-2 - Packager: Quadex Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 8, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purposes
- Uses
-
Warning
- For external use only.
- Flammable; keep away from fire or flame.
Do not use:
- in the eyes.
- over large areas of the body.
- longer than one week unless directed by a doctor.
- more than 3 times per day.
- if you are allergic to any ingredient in this product.
Ask a doctor if:
- used to treat deep or puncture wounds, animal bites, or serious burns.
- you are pregnant or nursing a baby.
-
Directions - Adults and children over 2 years of age
- Using only warm water and a washcloth, clean the lip area of any lip preparations, lotions, ointments, residual beverages, or cosmetics, including lipstick.
- Remove the cardboard cap from the applicator end and place the cardboard cap onto the glass/plastic vial end, opposite the brush end of the product.
- Squeeze the cardboard cap to break open the inner glass vial.
- Saturate the applicator end with solution by holding the brush end down and squeezing the container until you can see liquid on the brush applicator.
- For best results, massage the solution into the cold sore by rubbing. Rub firmly, but take care not to damage the tissue. The purpose of the rubbing is to deliver the drug to the infection site. Hold the vial so the solution flows to the sore.
- To treat most cold sores, usually one treatment is enough. If your symptoms go away and then return later, apply another dose for the second cold sore.
- Do not use more than 3 times per day.
- Children under 2 years of age — ask a doctor.
- Discard after use.
- Other information
- Inactive ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL - 0.6 ml Vial Blister Pack
-
INGREDIENTS AND APPEARANCE
BLISTER BLASTER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66774-1001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzalkonium chloride (UNII: F5UM2KM3W7) (Benzalkonium - UNII:7N6JUD5X6Y) Benzalkonium chloride 0.12 g in 100 mL Inactive Ingredients Ingredient Name Strength Isopropyl Alcohol (UNII: ND2M416302) 70 mL in 100 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66774-1001-2 2 in 1 BLISTER PACK 1 0.6 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333A 04/01/2010 Labeler - Quadex Pharmaceuticals, LLC (090438909) Establishment Name Address ID/FEI Business Operations James Alexander Corporation 040756421 MANUFACTURE