PEPAXTO- melphalan flufenamide injection, powder, lyophilized, for solution
Oncopeptides, AB
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PEPAXTO safely and effectively. See full prescribing information for PEPAXTO.
PEPAXTO® (melphalan flufenamide) for injection, for intravenous use Initial U.S. Approval: 2021 INDICATIONS AND USAGEPEPAXTO is an alkylating drug indicated in combination with dexamethasone, for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and one CD38-directed monoclonal antibody. (1) This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1) Limitations of Use: PEPAXTO is not indicated and is not recommended for use as a conditioning regimen for transplant outside of controlled clinical trials. (1, 5.5) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSFor injection: 20 mg melphalan flufenamide as a lyophilized powder in single-dose vial for reconstitution and dilution. (3) CONTRAINDICATIONSHistory of serious hypersensitivity reaction to melphalan flufenamide or melphalan. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (> 20%) are fatigue, nausea, diarrhea, pyrexia and respiratory tract infection. (6.1) Most common laboratory abnormalities (≥50%) are leukocytes decrease, platelets decrease, lymphocytes decrease, neutrophils decrease, hemoglobin decrease and creatinine increase. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Oncopeptides Inc at 1-866-522-8894 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 2/2021 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
PEPAXTO is indicated in combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and one CD38-directed monoclonal antibody.
This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s) [see Clinical Studies (14)].
Limitations of Use
PEPAXTO is not indicated and is not recommended for use as a conditioning regimen for transplant outside of controlled clinical trials [see Warnings and Precautions (5.5)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of PEPAXTO is 40 mg administered intravenously over 30 minutes on Day 1 of each 28-day cycle until disease progression or until unacceptable toxicity. Administer dexamethasone 40 mg orally or intravenously on Days 1, 8, 15 and 22 of each cycle. For patients 75 years of age or older, reduce the dose of dexamethasone to 20 mg. Refer to the prescribing information for dexamethasone for additional dosing information [see Clinical Studies (14)].
2.2 Recommended Premedication and Concomitant Medications
Consider providing a serotonin-3 (5-HT3) receptor antagonist or other antiemetics prior to and during the treatment with PEPAXTO.
2.3 Dosage Modifications for Adverse Reactions
Withold PEPAXTO if the neutrophil count is less than 1 × 109/L or the platelet count is less than 50 × 109/L.
The recommended dose reductions and dosage modifications for adverse reactions for PEPAXTO are presented in Table 1 and Table 2, respectively.
| Dose Reduction | Dosage* |
|---|---|
| First | 30 mg |
| Second | 20 mg |
| Subsequent | Permanently discontinue PEPAXTO in patients who are unable to tolerate 20 mg. |
| Adverse Reaction | Severity | Dosage Modification |
|---|---|---|
| Myelosuppression [see Warnings and Precautions (5.1, 5.2)] | Platelet count less than 50 × 109/L on an intended PEPAXTO dosing day |
|
| Absolute neutrophil count less than 1 × 109/L on an intended PEPAXTO dosing day |
|
|
| Grade 4 hematological adverse reaction on an intended PEPAXTO dosing day in 2 consecutive cycles |
|
|
| Non-Hematologic Adverse Reaction [see Adverse Reactions (6.1)] | Grade 2 |
|
| Grade 3 or 4 |
|
2.4 Preparation and Administration
PEPAXTO is a hazardous drug. Follow applicable special handling and disposal procedures.1
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if visibly opaque particles, discoloration or foreign particles are observed.
Reconstitute and dilute PEPAXTO prior to infusion.
Additional agents required for preparation:
- 5% Dextrose Injection, USP (room temperature)
- 250 mL bag of cold (2°C to 8°C / 36°F to 46°F) 0.9% Sodium Chloride Injection, USP (refrigerate for at least 4 hours)
Preparation Steps:
Read the complete instructions prior to starting preparation.
Steps 3 to 5 must be completed within 30 minutes.
| Reconstitution and dilution steps |
| Step 1
Determine the dose, the total volume of reconstituted PEPAXTO solution required, and the number of PEPAXTO vials needed. More than one vial may be needed for a full dose. Place PEPAXTO vial(s) at room temperature for at least 30 minutes. |
| Step 2
Shake the vial(s) vigorously or vortex to disintegrate the lyophilized PEPAXTO powder cake into a loose powder. |
| Step 3 to 5 must be completed within 30 minutes |
| Step 3
Aseptically reconstitute each vial with 40 mL of 5% Dextrose Injection, USP to obtain a final concentration of 0.5 mg/mL. Ensure the 5% Dextrose Injection, USP is room temperature (20°C to 25°C / 68°F to 77°F). Shake the vial(s) vigorously until solution is clear. Let the vial(s) stand to allow air bubbles to dissipate to confirm a clear solution. |
| Step 4
Withdraw 80 mL from a refrigerated (2°C to 8°C / 36°F to 46°F) 250 mL infusion bag of 0.9% Sodium Chloride Injection, USP. Discard the withdrawn 80 mL. |
| Step 5
Withdraw the required volume of reconstituted solution from the PEPAXTO vial(s) and transfer into an intravenous bag containing 0.9% Sodium Chloride Injection, USP to obtain a final concentration of 0.1 mg/mL to 0.16 mg/mL. Discard any unused portion left in the vial(s). Gently invert the bag to mix the solution. Do not shake. Check that the PEPAXTO solution is clear and colorless to pale yellow. Do not use if solution discoloration or particles are observed. |
Storage timelines:
PEPAXTO degrades in solution, especially at room temperature, and the storage timelines for diluted solution should not be exceeded:
| For immediate administration: |
| Infusion of the diluted PEPAXTO solution must begin within 60 minutes of start of reconstitution (step 3). |
| For delayed administration: |
| If not used for immediate administration, the diluted PEPAXTO solution should be placed in a refrigerator (2°C to 8°C / 36°F to 46°F) within 30 minutes after initial reconstitution (step 3) and store for up to 6 hours. |
Administration:
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if visibly opaque particles, discoloration or foreign particles are observed.
| Administration steps |
| Step 6
Administer PEPAXTO as a 30-minute intravenous infusion via a central venous access device, for example mediport, PICC or tunneled central venous catheter. If the infusion bag has been stored in a refrigerator, allow to reach to room temperature (20°C to 25°C / 68°F to 77°F). Start infusion within 30 minutes of removing the diluted PEPAXTO solution from the refrigerator. |
| Step 7
Administer PEPAXTO as an intravenous infusion via a central catheter over 30 minutes. |
| Step 8
Upon completion of PEPAXTO infusion, flush the central catheter per individual institutional guidelines. |
3 DOSAGE FORMS AND STRENGTHS
For Injection: 20 mg melphalan flufenamide as a sterile lyophilized white to off-white powder in a single-dose vial for reconstitution and further dilution.
4 CONTRAINDICATIONS
PEPAXTO is contraindicated in patients with a history of serious hypersensitivity reaction to melphalan flufenamide or melphalan [see Adverse Reactions (6.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Thrombocytopenia
Thrombocytopenia was reported in 99% of 157 patients who received PEPAXTO with dexamethasone. Grade 3 thrombocytopenia was reported in 26% and Grade 4 thrombocytopenia was reported in 54% of patients [see Adverse Reactions (6.1)]. Thrombocytopenia may lead to hemorrhage. Any Grade hemorrhage was reported in 28% of 157 patients. Grade 3 hemorrhage was reported in 3.2% and Grade 4 hemorrhage was reported in <1% of patients [see Adverse Reactions (6.1)].
Grade 3 or 4 thrombocytopenia occurred in 43% of patients during the first cycle, with a median time to onset of 15 days from the first dose.
Monitor platelets at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment with PEPAXTO. Do not administer PEPAXTO if the platelet count is less than 50 × 109/L. Withhold PEPAXTO until platelet count 50 × 109/L or greater and resume at same or reduced dose based on duration of interruption. Adjust dose and/or dose schedule based on signs and symptoms of bleeding [see Dosage and Administration (2.3)].
5.2 Neutropenia
Neutropenia was reported in 95% of 157 patients who received PEPAXTO with dexamethasone. Grade 3 neutropenia was reported in 41% and Grade 4 neutropenia was reported in 40% of patients. Febrile neutropenia was reported in 6% of patients [see Adverse Reactions (6.1)]. Neutropenia may lead to infection.
Grade 3 or 4 neutropenia occurred in 50% during the first cycle, with a median time to onset of 15 days from the first dose.
Monitor neutrophil counts at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment with PEPAXTO. Do not administer PEPAXTO if absolute neutrophil count less than 1 × 109/L. Withhold PEPAXTO until absolute neutrophil count is 1 × 109/L or greater and resume at same or reduced dose based on duration of interruption [see Dosage and Administration (2.3)]. Consider leukocyte growth factor as clinically appropriate.
5.3 Anemia
Anemia was reported in 84% of 157 patients who received PEPAXTO with dexamethasone. Grade 3 anemia was reported in 50% of 157 patients [see Adverse Reactions (6.1)].
Monitor red blood cell counts at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment with PEPAXTO. Treat anemia as clinically indicated and as per standard guidelines. Dosage modification and dose delay of PEPAXTO may be required to allow for recovery of red blood cells.
5.4 Infections
Fatal infections were reported in <1% of 157 patients who received PEPAXTO with dexamethasone. Any Grade infection was reported in 58% of 157 patients who received PEPAXTO and dexamethasone. Grade 3 infections were reported in 20% and Grade 4 infection was reported in 1.9% of patients. Respiratory tract infection occurred in 24% (Grade ≥3 in 5%), pneumonia in 13% (Grade ≥3 in 11%), and sepsis in 3.8% (Grade ≥3 in 3.2%) of patients [see Adverse Reactions (6.1)]. Consider antimicrobials as clinically appropriate.
5.5 Increased Risk of Mortality with PEPAXTO at Dosages Higher than the Recommended Dosage
A nonclinical safety study in dogs with melphalan flufenamide at dosages exceeding the recommended dose for relapsed or refractory multiple myeloma was associated with mortality [see Nonclinical Toxicology (13.2)]. There is limited clinical experience of PEPAXTO at dosages higher than recommended. The safety and efficacy of PEPAXTO has not been established for use as a conditioning regimen in patients receiving transplant.
5.6 Secondary Malignancies
Secondary malignancies such as myelodysplastic syndromes or acute leukemia have occurred in patients with multiple myeloma who have received PEPAXTO. Monitor patients long-term for the development of secondary malignancies.
5.7 Embryo-Fetal Toxicity
Based on its mechanism of action, PEPAXTO can cause fetal harm when administered to a pregnant woman because it is genotoxic and targets actively dividing cells. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with PEPAXTO and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with PEPAXTO and for 3 months after the last dose [see Use In Specific Populations (8.1, 8.3)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Thrombocytopenia [see Warnings and Precautions (5.1)].
- Neutropenia [see Warnings and Precautions (5.2)].
- Anemia [see Warnings and Precautions (5.3)].
- Infections [see Warnings and Precautions (5.4)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Relapsed Refractory Multiple Myeloma (RRMM)
The safety of PEPAXTO was evaluated in HORIZON [see Clinical Studies (14)]. Patients received PEPAXTO 40 mg intravenously on Day 1 of each 28-day cycle, in combination with dexamethasone 40 mg orally (or 20 mg for patients 75 years and older) on Days 1, 8, 15 and 22 of each cycle (N=157). Patients were enrolled if they had absolute neutrophil count of 1 × 109/L or higher and platelet count of 75 × 109/L or greater. Among patients who received PEPAXTO, 29% were exposed for 6 months or longer and 6% were exposed for greater than one year.
Serious adverse reactions occurred in 49% of patients who received PEPAXTO. Serious adverse reactions in >3% of patients included pneumonia (10%), respiratory tract infection (6%), thrombocytopenia (5%), febrile neutropenia (5%) and sepsis (3.2%). Fatal adverse reactions occurred in 10 patients (6%) who received PEPAXTO, where general physical health deterioration (1.9%) and respiratory failure (1.3%) represented more than 1%.
Permanent discontinuation of PEPAXTO due to an adverse reaction occurred in 22% of patients. Adverse reactions which resulted in permanent discontinuation of PEPAXTO in >3% of patients included thrombocytopenia (11%).
Dosage interruptions of PEPAXTO due to an adverse reaction occurred in 62% of patients. The adverse reactions which resulted in dosage interruption of PEPAXTO in >3% of patients included thrombocytopenia (43%), neutropenia (29%), anemia (10%), respiratory tract infection (7%), leukopenia (6%) and pyrexia (4.5%).
Dose reductions of PEPAXTO due to an adverse reaction occurred in 27% of patients. Adverse reactions which resulted in dose reductions of PEPAXTO in >3% patients included thrombocytopenia (22%) and neutropenia (6%).
The most common adverse reactions (≥20%) were fatigue, nausea, diarrhea, pyrexia and respiratory tract infection. The most common laboratory abnormalities (≥50%) were leukocytes decreased, platelets decreased, lymphocytes decreased, neutrophils decreased, hemoglobin decreased and creatinine increased.
Table 3 summarizes the adverse reactions in HORIZON.
| Adverse Reaction | PEPAXTO with Dexamethasone (N=157) |
|
|---|---|---|
| All Grades (%) | Grade 3 or 4 (%) |
|
|
||
| General disorders and administration site disorders | ||
| Fatigue* | 55 | 6 |
| Pyrexia† | 24 | 1.9 |
| Edema peripheral† | 14 | 1.3 |
| Gastrointestinal disorders | ||
| Nausea† | 32 | 0.6 |
| Diarrhea | 27 | 0 |
| Constipation† | 15 | 0.6 |
| Vomiting | 13 | 0 |
| Infections | ||
| Respiratory tract infection†,‡ | 24 | 5 |
| Pneumonia§ | 13 | 11 |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough† | 17 | 0 |
| Dyspnea† | 11 | 1.3 |
| Dyspnea exertional | 10 | 0 |
| Metabolism and nutrition disorders | ||
| Decreased appetite† | 14 | 0.6 |
| Hypokalemia† | 14 | 1.3 |
| Hypocalcemia† | 10 | 0.6 |
| Nervous system disorders | ||
| Headache | 13 | 0 |
| Dizziness | 11 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Bone pain† | 13 | 1.9 |
| Pain in extremity† | 13 | 1.9 |
| Back pain† | 12 | 0.6 |
| Arthralgia | 10 | 0 |
| Psychiatric disorders | ||
| Insomnia† | 11 | 0.6 |
Clinically relevant adverse reactions in <10% of patients who received PEPAXTO in combination with dexamethasone (N=157) included:
Allergic conditions: hypersensitivity reaction (7%)
Blood and lymphatic system disorders: febrile neutropenia (6%)
Infections: sepsis (3.8%)
Hemorrhages: Grade 3 or 4 hemorrhages (3.8%)
Table 4 summarizes the laboratory abnormalities in HORIZON.
| Laboratory Abnormality | PEPAXTO with Dexamethasone* | |
|---|---|---|
| All Grades†
(%) | Grade 3- 4‡
(%) |
|
| Leukocytes decrease | 99 | 88 |
| Platelets decrease | 99 | 80 |
| Lymphocytes decrease | 97 | 95 |
| Neutrophils decrease | 95 | 82 |
| Hemoglobin decrease | 84 | 50 |
| Creatinine increase | 68 | 1§ |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action [see Clinical Pharmacology (12.1)], PEPAXTO can cause fetal harm when administered to a pregnant woman. There are no available data on PEPAXTO use in pregnant women to evaluate for a drug-associated risk. PEPAXTO is a genotoxic drug [see Nonclinical Toxicology (13.1)]. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There is no data on the presence of melphalan flufenamide or its metabolites in human breast milk, or the effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with PEPAXTO and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
PEPAXTO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating PEPAXTO.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with PEPAXTO and for 6 months after the last dose.
Males
Based on genotoxicity findings, advise males with female partners of reproductive potential to use effective contraception during treatment with PEPAXTO and for 3 months after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Males
Based on findings of melphalan flufenamide in animals, PEPAXTO may impair male fertility [see Nonclinical Toxicology (13.1)]. Alkylating drugs, such as PEPAXTO, can also cause irreversible testicular suppression in patients.
8.4 Pediatric Use
The safety and effectiveness of PEPAXTO have not been established in pediatric patients.
8.5 Geriatric Use
Of the 157 patients with RRMM who received PEPAXTO, 50% were 65 years and older, while 16% were 75 years and older. No overall differences in safety were observed between these patients and younger patients. Clinical studies of PEPAXTO in patients with RRMM did not include sufficient numbers of patients 65 years of age and older to determine if they respond differently from younger adult patients.
8.6 Renal Impairment
No dose adjustment of PEPAXTO is recommended in patients with creatinine clearance (CLcr) 45 to 89 mL/min calculated using Cockcroft-Gault equation [see Clinical Pharmacology (12.3)]. PEPAXTO has not been studied in patients with CLcr 15 to 44 mL/min.
11 DESCRIPTION
Melphalan flufenamide is an alkylating drug. The chemical name is Ethyl (2S)-2-[[(2S)-2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoyl]amino]-3-(4-fluorophenyl)propanoate hydrochloride and the molecular weight is 498.4 as free base and 534.9 as the hydrochloride salt. The structural formula is:
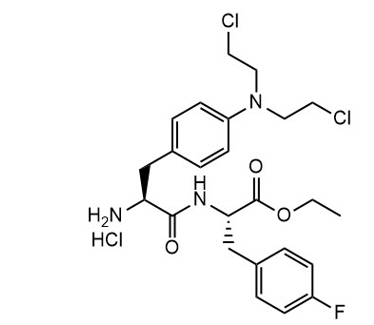
Melphalan flufenamide hydrochloride is soluble in most organic solvents, while sparsely soluble in aqueous solutions. The pKa value is 7.13.
PEPAXTO for injection is supplied as a sterile, white to off-white lyophilized powder in a single-dose vial for intravenous use. Each vial contains 20 mg melphalan flufenamide (equivalent to 21.48 mg melphalan flufenamide hydrochloride) and 1,000 mg sucrose.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Melphalan flufenamide is a peptide conjugated alkylating drug. Due to its lipophilicity, melphalan flufenamide is passively distributed into cells and thereafter enzymatically hydrolyzed to melphalan. Similar to other nitrogen mustard drugs, cross-linking of DNA is involved in the antitumor activity of melphalan flufenamide. In cellular assays, melphalan flufenamide inhibited proliferation and induced apoptosis of hematopoietic and solid tumor cells. Additionally, melphalan flufenamide showed synergistic cytotoxicity with dexamethasone in melphalan resistant and non-resistant multiple myeloma cell lines.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of PEPAXTO have not been fully characterized.
12.3 Pharmacokinetics
Melphalan flufenamide peak plasma concentrations were reached during the 30-minute infusion. Peak plasma concentrations of the active metabolite melphalan were reached 4 to 15 minutes after the end of infusion of PEPAXTO 40 mg. Following PEPAXTO 40 mg, the mean (CV%) Cmax was 432 ng/mL (30%) and AUC0-INF was 3,143 µg/mL∙hr (28%) for melphalan after a single dose. The mean (CV%) Cmax was 419 ng/mL (33%) and AUC0-INF was 2,933 µg/mL∙hr (29%) for melphalan at steady-state.
Distribution
In vivo the disappearance of melphalan flufenamide from plasma is rapid and is attributed to distribution to peripheral tissues with no late redistribution back to plasma.
The mean (CV%) volume of distribution was 35 L (71%) for melphalan flufenamide and 76 L (32%) for melphalan after a single dose.
Elimination
After the end of infusion of PEPAXTO 40 mg, the mean (CV%) elimination half-life of melphalan flufenamide is 2.1 minutes (34%). The mean (CV%) elimination half-life of melphalan is 70 minutes (21%). The mean (CV%) clearance of melphalan flufenamide and melphalan is 692 L/hr (49%) and 23 L/hr (23%), respectively, at the recommended dosage of PEPAXTO 40 mg.
Specific Populations
Higher melphalan exposures were observed in patients with lower body surface area. No clinically meaningful differences in the PK of melphalan were observed based on age (35 to 85 years old), renal impairment (CLcr 45 to 89 mL/min) and mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN, or total bilirubin 1 to 1.5 × ULN and any AST).
The effect of sex, race/ethnicity, moderate to severe hepatic impairment (total bilirubin >1.5 × ULN and any AST), and renal impairment (CLcr 15 to 44 mL/min) on melphalan flufenamide and melphalan PK is unknown.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted with melphalan flufenamide.
PEPAXTO is genotoxic. In studies conducted in vitro, melphalan flufenamide caused irreversible DNA damage.
Repeat-dose toxicity studies with melphalan flufenamide in animals showed adverse effects on male reproductive organs. Melphalan flufenamide was administered intravenously to rats at 20, 40, or 55 mg/m2, and to dogs at 0.45 or 0.90 mg/kg (9 or 18 mg/m2) every 21 days for two or three doses. Decreased testes weights and depletion of germ cells were observed in both species, and epididymal oligospermia was observed in dogs. Adverse effects on male reproductive organs were observed in dogs at dose levels less than the recommended clinical dose of 40 mg. The reversibility of adverse effects on male reproductive organs was not assessed.
13.2 Animal Toxicology and/or Pharmacology
Dogs were intravenously administered a single dose of melphalan flufenamide (17.5 mg/kg) or an equimolar dose of melphalan; these dose levels were representative of dosages needed for myeloablation. Increased mortality was observed in dogs administered melphalan flufenamide despite similar melphalan exposure in animals administered melphalan flufenamide or melphalan.
14 CLINICAL STUDIES
The efficacy of PEPAXTO in combination with dexamethasone was evaluated in HORIZON [NCT02963493], a multicenter, single-arm trial. Eligible patients were required to have relapsed or refractory multiple myeloma. Patients received PEPAXTO 40 mg intravenously on Day 1 and dexamethasone 40 mg orally (20 mg for patients ≥75 years of age) on Day 1, 8, 15 and 22 of each 28-day cycle until disease progression or unacceptable toxicity.
A total of 157 patients accepting a central venous catheter and with estimated creatinine clearance by Cockcroft-Gaut formula ≥45 mL/min were enrolled. Patients with primary refractory disease (i.e. never responded with at least minimal response to any prior treatment) were excluded. Ninety seven patients had received four or more prior lines of therapies and were refractory to at least one proteasome inhibitor, at least one immunomodulatory agent and a CD38-directed monoclonal antibody. The median age was 65 years (range: 35 to 86 years); 58% were male, 87% were White and 6% were Black or African American. Disease characteristics in these 97 patients are summarized in Table 5.
The major efficacy outcome measure was overall response rate (ORR) and Duration of Response (DoR) assessed according to the International Myeloma Working Group (IMWG) Criteria by investigators. Efficacy results in the 97 patients are provided in Table 6. The median time to first response was 2.1 months (range: 1.0 to 6.1 months).
| Parameter | PEPAXTO with Dexamethasone (N=97) |
|---|---|
|
|
| Years from diagnosis to start of PEPAXTO, median (range) | 6.4 (2.1 to 24.6) |
| Prior treatment regimens, median (range) | 6 (4 to 12) |
| Documented refractory status, (%) | |
| Lenalidomide | 94 |
| Pomalidomide | 92 |
| Bortezomib | 74 |
| Carfilzomib | 63 |
| Daratumumab | 93 |
| Alkylator refractory, (%) | 75 |
| Previous stem cell transplant, (%) | 70 |
| International Staging System at Baseline, (%) | |
| I | 30 |
| II | 32 |
| III | 34 |
| Missing/Unknown | 4 |
| High-risk cytogenetics*, (%) | 33 |
| Extramedullary disease (EMD), (%) | 41 |
| PEPAXTO with Dexamethasone (N=97) |
|
|---|---|
| Overall response rate (ORR), n (%) (95% CI) | 23 (23.7) (15.7, 33.4) |
| Stringent complete response (sCR) | 0 |
| Complete Response (CR) | 0 |
| Very good partial response (VGPR), n (%) | 9 (9.3) |
| Partial response (PR), n (%) | 14 (14.4) |
| Median duration of response in months (95% CI) | 4.2 (3.2, 7.6) |
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
PEPAXTO is a white to off-white lyophilized powder for reconstitution (after reconstitution the solution is clear and colorless to light yellow) supplied in a 50 mL single dose vial containing 20 mg melphalan flufenamide. Each 20 mg vial is packaged in a single carton (NDC 73657-020-01).
The vial stopper is not manufactured with natural rubber latex.
Storage
Store refrigerated at 2°C to 8°C (36°F to 46°F) and protect from light. Retain in original carton until use.
Handling and Disposal
PEPAXTO is a hazardous drug. Follow special handling and disposal procedures.1 All materials that have been utilized for dilution and administration, including any reconstituted solution made over 30 minutes prior, should be disposed of according to standard procedures for hazardous drugs.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Thrombocytopenia, Neutropenia and Anemia
- Advise patients that PEPAXTO can cause myelosuppression. Advise patients to immediately report signs or symptoms of thrombocytopenia (bleeding and easy bruising), neutropenia (symptoms of infection, such as fever, chills, cough, pain, or burning during urination) and anemia (fatigue and shortness of breath) to their healthcare provider.
- Advise patients that complete blood counts will be monitored at baseline, during treatment, and as clinically indicated [see Warnings and Precautions (5.1, 5.2, 5.3)].
Infections
Advise patients that PEPAXTO can cause infections. Instruct patients to immediately report new or worsening signs or symptoms (e.g., chills, fever) of infection to their healthcare provider [see Warnings and Precautions (5.4)].
Secondary Malignancies
Advise patients on the risk of second primary malignancies [see Warnings and Precautions (5.6)].
Embryo-Fetal Toxicity
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with PEPAXTO and 6 months after the last dose [see Use in Specific Populations (8.3)].
- Advise males with female partners of reproductive potential to use effective contraception during treatment with PEPAXTO and for 3 months after the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with PEPAXTO and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Manufactured for: Oncopeptides AB (publ), Stockholm, Sweden.
Distributed by: Oncopeptides Inc. 200 Fifth Avenue, Suite #1030 Waltham, MA 02451, USA
PEPAXTO is a registered trademark of Oncopeptides AB (publ)
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued: 02/2021 |
| PATIENT INFORMATION
PEPAXTO (peh-PAX-toe) (melphalan flufenamide) for injection, for intravenous use |
|
| What is PEPAXTO? | |
| PEPAXTO is a prescription medicine used in combination with the medicine dexamethasone to treat adults with multiple myeloma who did not respond to or stopped responding to at least four prior medicines including at least one proteasome inhibitor, one immunomodulatory agent and one CD38-directed antibody. PEPAXTO is not for use to prepare for transplant. It is not known if PEPAXTO is safe and effective in children. |
|
| Do not receive PEPAXTO if you have a history of a severe allergic reaction to melphalan flufenamide or melphalan. | |
| Before receiving PEPAXTO, tell your healthcare provider about all of your medical conditions, including if you: | |
|
|
| How will I receive PEPAXTO? | |
|
|
| What are the possible side effects of PEPAXTO? | |
| PEPAXTO may cause serious side effects, including: | |
PEPAXTO may cause fertility problems in males and females, which may affect your ability to have children. Talk with your healthcare provider if you have concerns about fertility. The most common side effects of PEPAXTO include, low blood cell counts, fatigue, nausea, diarrhea, fever, and cold-like symptoms (respiratory tract infection). These are not all of the possible side effects of PEPAXTO. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088. |
|
| General information about the safe and effective use of PEPAXTO. | |
| Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about PEPAXTO that is written for health professionals. | |
| What are the ingredients in PEPAXTO? | |
| Active ingredient: melphalan flufenamide | |
| Inactive ingredient: sucrose | |
| Manufactured for Oncopeptides AB (publ), Stockholm, Sweden. | |
| Marketed and distributed by Oncopeptides Inc., 200 Fifth Avenue, Suite #1030 Waltham, MA 02451, USA. | |
| PEPAXTO is a registered trademark of Oncopeptides AB (publ). | |
| For more information, go to www.PEPAXTO.com or call 1-866-522-8894. | |
| PEPAXTO
melphalan flufenamide injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Oncopeptides, AB (632402728) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Magle Chemoswed AB | 559533158 | API MANUFACTURE(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cenexi-Laboratoires Thissen SA (Cenexi LT) | 370088959 | MANUFACTURE(73657-020) , ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotek Produktion & Laboratorier AB (APL) | 632474078 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Biopharma Product Testing, Denmark A/S (Eurofins) | 311900950 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Solvias AG (Solvias) | 480739627 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmascience Inc. | 202657094 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc.; "PCI of Illinois" | 098908572 | LABEL(73657-020) , PACK(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West Pharmaceutical Services, Inc. | 002330983 | ANALYSIS(73657-020) | |
