Label: DOCUSATE CALCIUM 240 MG SODIUM FREE- docusate calcium capsule, liquid filled
- NDC Code(s): 50268-266-11, 50268-266-15
- Packager: AvPAK
- This is a repackaged label.
- Source NDC Code(s): 54629-640
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 31, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
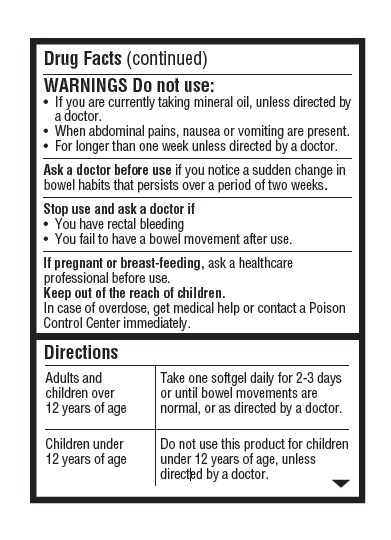
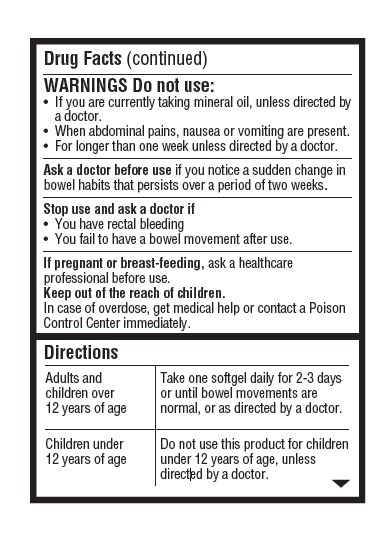
- Warnings: Do not use
- Ask a Doctor Before Use
- Stop Use and Ask a Doctor If
- If Pregnant or Breast Feeding
- Keep Out of Reach of Children.
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DOCUSATE CALCIUM 240 MG SODIUM FREE
docusate calcium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50268-266(NDC:54629-640) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE CALCIUM (UNII: 6K7YS503HC) (DOCUSATE - UNII:M7P27195AG) DOCUSATE CALCIUM 240 mg Inactive Ingredients Ingredient Name Strength CORN OIL (UNII: 8470G57WFM) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color red Score no score Shape OVAL Size 15mm Flavor Imprint Code NV02 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50268-266-15 50 in 1 BOX 05/17/2017 1 NDC:50268-266-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 05/17/2017 Labeler - AvPAK (832926666)