BDR BIO CELL MASK- honey, hyaluronic acid patch
Goldeneye Permanent System Gmbh Germany
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
GOLDENEYE - bdr Bio cell mask
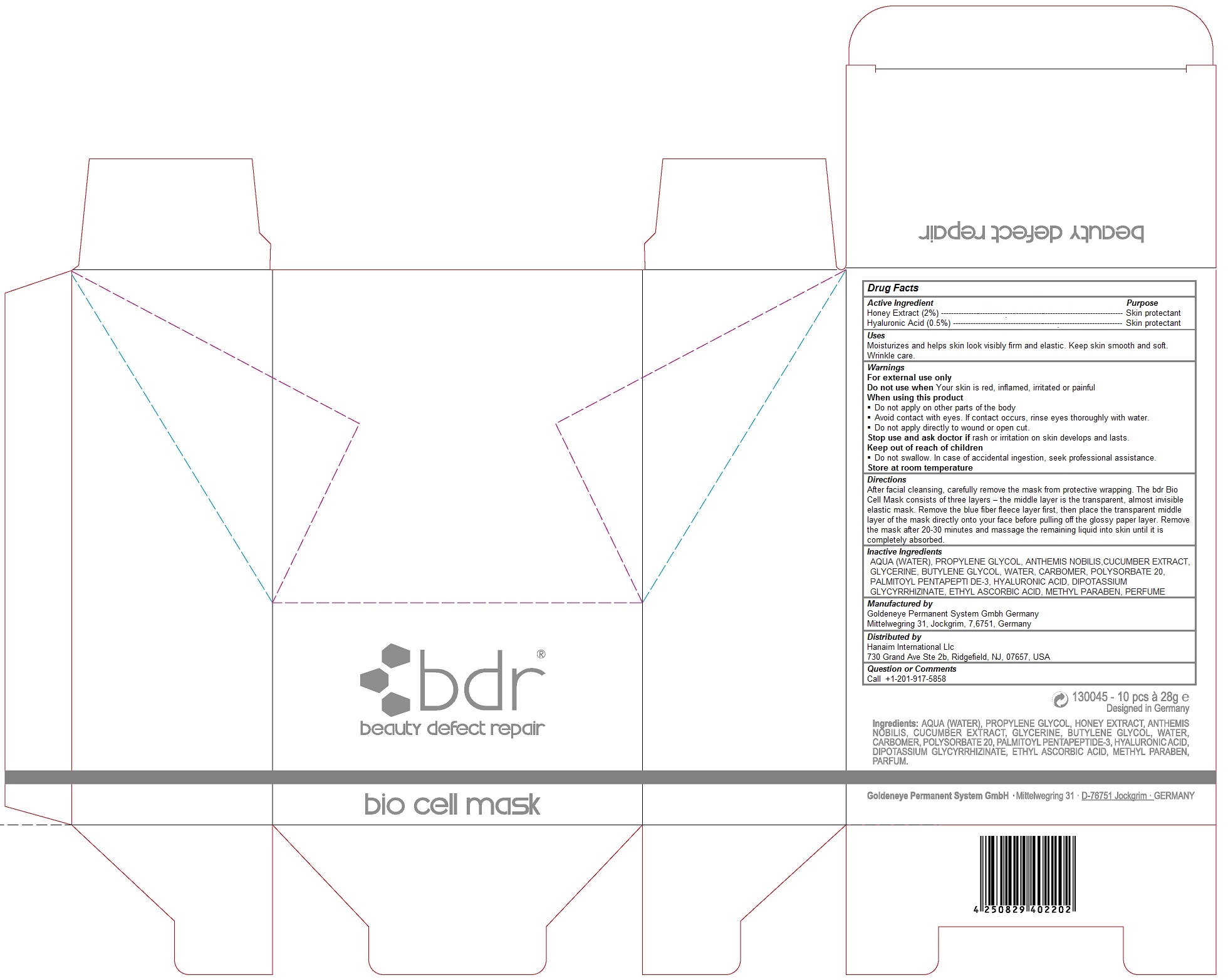
Keep out of reach of children
Do not swallow. In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Warnings
For external use only
Do not use when Your skin is red, inflamed, irritated or painful
When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on skin develops and lasts.
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
Store at room temperature
Uses
Moisturizes and helps skin look visibly firm and elastic. Keep skin smooth and soft. Wrinkle care.
Directions
After facial cleansing, carefully remove the mask from protective wrapping. The bdr Bio Cell Mask consists of three layers – the middle layer is the transparent, almost invisible elastic mask. Remove the blue fiber fleece layer first, then place the transparent middle layer of the mask directly onto your face before pulling off the glossy paper layer. Remove the mask after 20-30 minutes and massage the remaining liquid into skin until it is completely absorbed.
| BDR BIO CELL MASK
honey, hyaluronic acid patch |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Goldeneye Permanent System Gmbh Germany (329178144) |
| Registrant - Hanaim International Llc (070319425) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Goldeneye Permanent System Gmbh Germany | 329178144 | manufacture(71056-113) | |