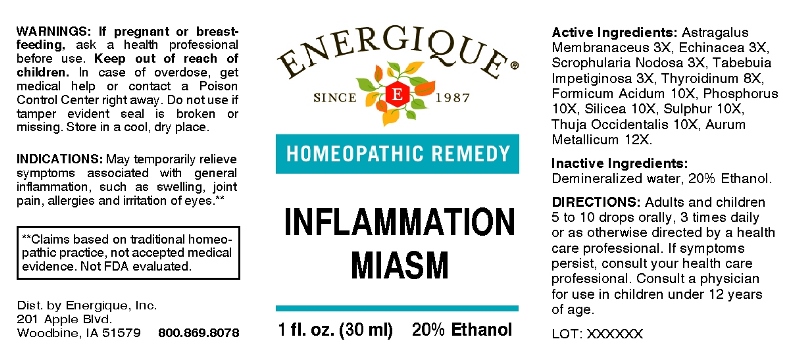
INFLAMMATION MIASM- astragalus membranaceus, echinacea (angustifolia), scrophularia nodosa, tabebuia impetiginosa, thyroidinum (suis), formicum acidum, phosphorus, silicea, sulphur, thuja occidentalis, aurum metallicum liquid
Energique, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Astragalus Membranaceus 3X, Echinacea (Angustifolia) 3X, Scrophularia Nodosa 3X, Tabebuia Impetiginosa 3X, Thyroidinum (Suis) 8X, Formicum Acidum 10X, Phosphorus 10X, Silicea 10X, Sulphur 10X, Thuja Occidentalis 10X, Aurum Metallicum 12X.
INDICATIONS:
May temporarily relieve symptoms associated with general inflammation, such as swelling, joint pain, allergies and irritation of eyes.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.
| INFLAMMATION MIASM
astragalus membranaceus, echinacea (angustifolia), scrophularia nodosa, tabebuia impetiginosa, thyroidinum (suis), formicum acidum, phosphorus, silicea, sulphur, thuja occidentalis, aurum metallicum liquid |
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
| Labeler - Energique, Inc. (789886132) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(44911-0229) , api manufacture(44911-0229) , label(44911-0229) , pack(44911-0229) | |