Label: ATOPALM ACNE CONTROLLING- salicylic acid lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 51141-7000-1 - Packager: NeoPharm Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 9, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Use
- Warnings
-
When using this product
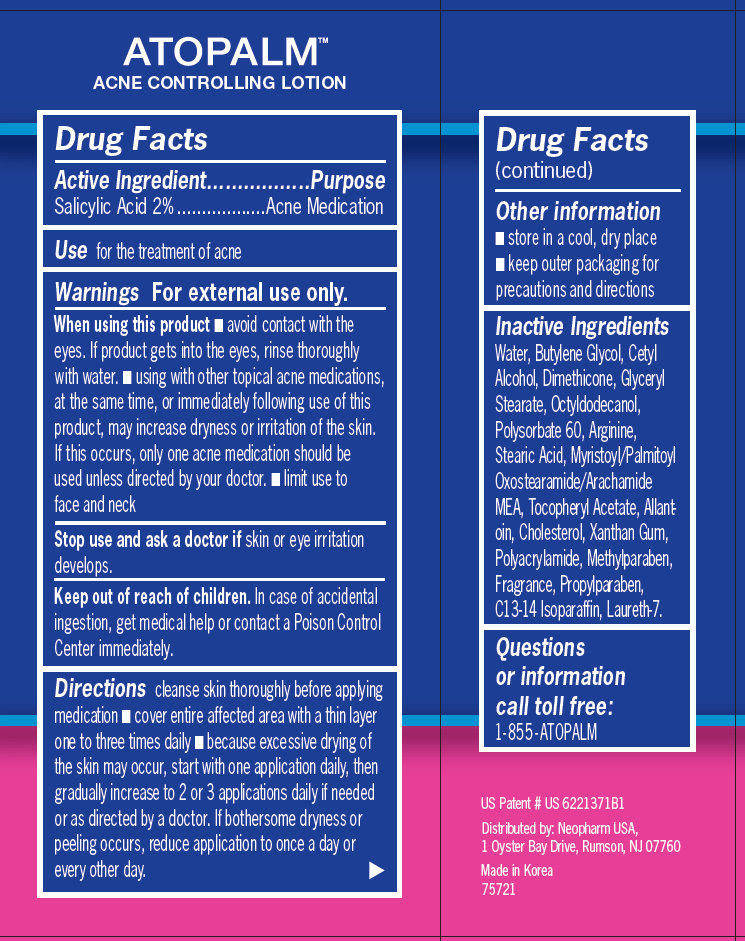
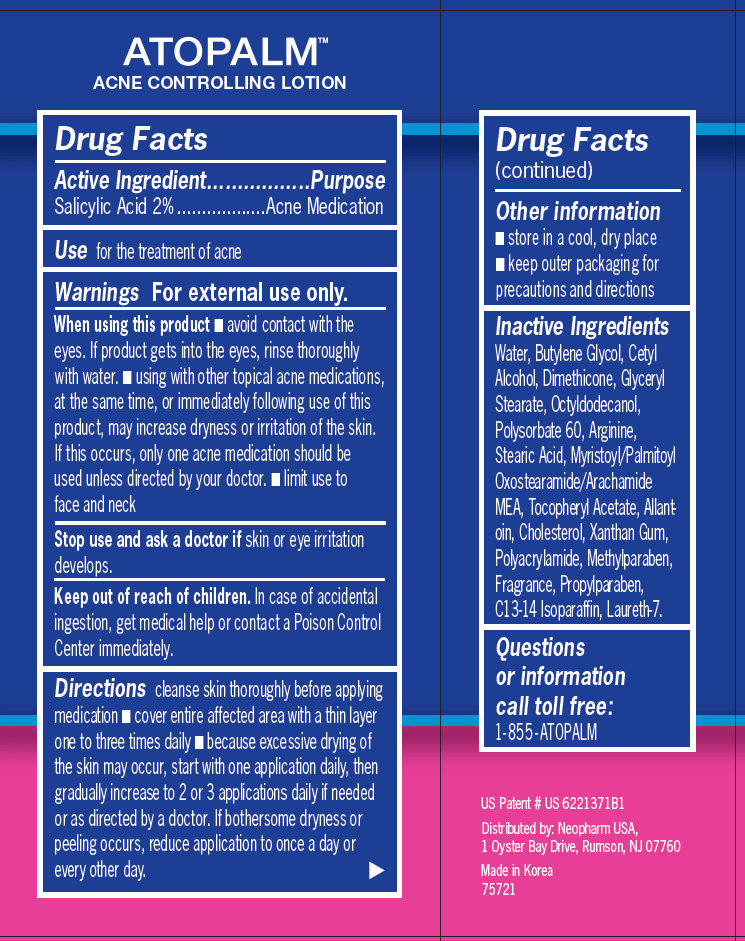
- avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water. - using with other topical acne medications, at the same time, or immediately following use of this product, may increase dryness or irritation of the skin. If this occurs, only one acne medication should be used unless directed by your doctor. - limit use to face and neck
- Stop use and ask a doctor
- Keep out of reach of children
- Ask Doctor
-
Directions
cleanse skin thoroughly before applying medication - cover entire affected area with a thin layer one to three times daily - because excessive drying of the skin may occur, start with one application daily, then gradually increase to 2 or 3 applications daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other information
-
Inactive Ingredients
Water, Butylene Glycol, Cetyl Alcohol, Dimethicone, Glyceryl Stearate, Octyldodecanol, Polysorbate 60, Arginine, Stearic Acid, Myristoyl/Palmitoyl Oxostearamide/Arachamide MEA, Tocopheryl Acetate, Allantoin, Cholesterol, Xanthan Gum, Polyacrylamide, Methylparaben, Fragrance, Propylparaben, C13-14 Isoparaffin, Laureth-7.
- Questions or information
- Purpose
- Description
-
PRINCIPAL DISPLAY PANEL
ATOPALM ACNE CONTROLLING LOTION With US Patented MLE Technology With 2% Salicylic Acid-Acne Medication to help reduce the appearance of redness and pimple size overnight 1.3 fl oz /38.4 ml Drug Facts
Active Ingredient............................. ....................Purpose
Salicylic Acid 2% ................................. Acne Medication
Use for the treatment of acne Warnings For external use only. When using this product - avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water. - using with other topical acne medications, at the same time, or immediately following use of this product, may increase dryness or irritation of the skin. If this occurs, only one acne medication should be used unless directed by your doctor. - limit use to face and neck Stop use and ask a doctor if skin or eye irritation develops. Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Center immediately. Directions cleanse skin thoroughly before applying medication - cover entire affected area with a thin layer one to three times daily - because excessive drying of the skin may occur, start with one application daily, then gradually increase to 2 or 3 applications daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. Other information - store in a cool, dry place - keep outer packaging for precautions and directions Questions or information call toll free: 1-855-ATOPALM Distributed by: Neopharm USA, 1 Oyster Bay Drive, Rumson, NJ 07760 Made in Korea 75721
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
ATOPALM ACNE CONTROLLING LOTION Dermatologist Tested ATOPALM ACNE CONTROLLING LOTION ATOPALM ACNE CONTROLLING LOTION 2% Salicylic Acid Acne Medication Quick absorbing all over face lotion has a salicylic based formula that works through the night to help visibly reduce redness and reduce the size of existing pimples. With US Patented M L E Technology Helps Visibly Reduce Redness and Reduce Pimple Size Overnight 1.3 fl oz /38.4 ml 8 57811 00221 7
- Product Package Inner Label
- Product Package Outer Label 1
- Product Package Outer Label 2
-
INGREDIENTS AND APPEARANCE
ATOPALM ACNE CONTROLLING
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51141-7000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) OCTYLDODECANOL (UNII: 461N1O614Y) POLYSORBATE 60 (UNII: CAL22UVI4M) ARGININE (UNII: 94ZLA3W45F) STEARIC ACID (UNII: 4ELV7Z65AP) MYRISTOYL/PALMITOYL OXOSTEARAMIDE/ARACHAMIDE MEA (UNII: 1211AIM8G7) ALLANTOIN (UNII: 344S277G0Z) CHOLESTEROL (UNII: 97C5T2UQ7J) XANTHAN GUM (UNII: TTV12P4NEE) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600 KD) (UNII: 0L414VCS5Y) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) LAURETH-7 (UNII: Z95S6G8201) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51141-7000-1 1 in 1 CARTON 1 38.4 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 11/04/2011 Labeler - NeoPharm Co., Ltd. (965502912) Establishment Name Address ID/FEI Business Operations NeoPharm Co., Ltd. 631101883 manufacture