Label: GUANFACINE tablet, extended release
- NDC Code(s): 42291-324-01, 42291-325-01, 42291-326-01, 42291-327-01
- Packager: AvKARE
- This is a repackaged label.
- Source NDC Code(s): 0228-2850, 0228-2851, 0228-2853, 0228-2855
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
Guanfacine Extended-Release Tablets
Rx Only
These highlights do not include all the information needed to use GUANFACINE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for GUANFACINE EXTENDED-RELEASE TABLETS.
GUANFACINEextended-release tablets, for oral use
Initial U.S. Approval: 1986RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Recommended dose: 1 mg to 4 mg (0.05 mg/kg to 0.12 mg/kg target weight based dose range) once daily in the morning or evening based on clinical response and tolerability (
2.2).
- Begin at a dose of 1 mg once daily and adjust in increments of no more than 1 mg/week (
2.2).
- Do not crush, chew or break tablets before swallowing (
2.1).
- Do not administer with high-fat meals, because of increased exposure (
2.1).
- Do not substitute for immediate-release guanfacine tablets on a mg-per-mg basis, because of differing pharmacokinetic profiles (
2.3).
- If switching from immediate-release guanfacine, discontinue that treatment and titrate with guanfacine extended-release as directed (
2.3).
- When discontinuing, taper the dose in decrements of no more than 1 mg every 3 to 7 days to avoid rebound hypertension ( 2.5).
DOSAGE FORMS AND STRENGTHS
Extended-release tablets: 1 mg, 2 mg, 3 mg and 4 mg ( 3)
CONTRAINDICATIONS
History of hypersensitivity to guanfacine extended-release, its inactive ingredients, or other products containing guanfacine ( 4).
WARNINGS AND PRECAUTIONS
- Hypotension, bradycardia, syncope: Titrate slowly and monitor vital signs frequently in patients at risk for hypotension, heart block, bradycardia, syncope, cardiovascular disease, vascular disease, cerebrovascular disease or chronic renal failure. Measure heart rate and blood pressure prior to initiation of therapy, following dose increases, and periodically while on therapy. Avoid concomitant use of drugs with additive effects unless clinically indicated. Advise patients to avoid becoming dehydrated or overheated (
5.1).
- Sedation and somnolence: Occur commonly with guanfacine extended-release. Consider the potential for additive sedative effects with CNS depressant drugs. Caution patients against operating heavy equipment or driving until they know how they respond to guanfacine extended-release (
5.2).
- Cardiac Conduction Abnormalities: May worsen sinus node dysfunction and atrioventricular (AV) block, especially in patients taking other sympatholytic drugs. Titrate slowly and monitor vital signs frequently ( 5.3).
- Rebound Hypertension: Abrupt discontinuation of guanfacine extended-release tablets can lead to clinically significant and persistent rebound hypertension. Subsequent hypertensive encephalopathy was also reported. To minimize the risk of rebound hypertension upon discontinuation, the total daily dose of guanfacine extended-release tablets should be tapered in decrements of no more than 1 mg every 3 to 7 days ( 5.4).
ADVERSE REACTIONS
Most common adverse reactions (greater than or equal to 5% and at least twice placebo rate) in fixed-dose monotherapy ADHD trials in children and adolescents (6 to 17 years): hypotension, somnolence, fatigue, nausea, and lethargy ( 6.1)
Flexible dose-optimization ADHD trials in children (6 to 12 years): somnolence, hypotension, abdominal pain, insomnia, fatigue, dizziness, dry mouth, irritability, nausea, vomiting, and bradycardia ( 6.1).
Adjunctive treatment to psychostimulant ADHD trial in children and adolescents (6 to 17 years): somnolence, fatigue, insomnia, dizziness, and abdominal pain ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact AvKARE, Inc. at 1-855-361-3993 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Strong and moderate CYP3A4 inhibitors increase guanfacine exposure. Decrease guanfacine extended-release to 50% of target dosage when coadministered with strong and moderate CYP3A4 inhibitors ( 2.7).
- Strong and moderate CYP3A4 inducers decrease guanfacine exposure. Based on patient response, consider titrating guanfacine extended-release dosage up to double the target dosage over 1 to 2 weeks ( 2.7).
Additional pediatric use information for patients ages 6 to 17 years is approved for Shire US Inc.’s INTUNIV® (guanfacine) extended-release tablet product. However, due to Shire US Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2018
- Recommended dose: 1 mg to 4 mg (0.05 mg/kg to 0.12 mg/kg target weight based dose range) once daily in the morning or evening based on clinical response and tolerability (
2.2).
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Instruction for Use
2.2 Dose Selection
2.3 Switching from Immediate-Release Guanfacine to Guanfacine Extended-Release
2.4 Maintenance Treatment
2.5 Discontinuation of Treatment
2.6 Missed Doses
2.7 Dosage Adjustment with Concomitant Use of Strong and Moderate CYP3A4 Inhibitors or Inducers
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension, Bradycardia, and Syncope
5.2 Sedation and Somnolence
5.3 Cardiac Conduction Abnormalities
5.4 Rebound Hypertension
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Guanfacine extended-release tablets are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) as monotherapy and as adjunctive therapy to stimulant medications [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 General Instruction for Use
Swallow tablets whole. Do not crush, chew, or break tablets because this will increase the rate of guanfacine release. Do not administer with high fat meals, due to increased exposure.
2.2 Dose Selection
Take guanfacine extended-release tablets orally once daily, either in the morning or evening, at approximately the same time each day. Begin at a dose of 1 mg/day, and adjust in increments of no more than 1 mg/week.
In monotherapy-clinical trials, there was dose- and exposure-related clinical improvement as well as risks for several clinically significant adverse reactions (hypotension, bradycardia, sedative events). To balance the exposure-related potential benefits and risks, the recommended target dose range depending on clinical response and tolerability for guanfacine extended-release is 0.05 to 0.12 mg/kg/day (total daily dose between 1 mg to
4 mg).In the adjunctive trial which evaluated guanfacine extended-release treatment with psychostimulants, the majority of patients reached optimal doses in the 0.05 to 0.12 mg/kg/day range. Doses above 4 mg/day have not been studied in adjunctive trials.
Additional pediatric use information for patients ages 6 to 17 years is approved for Shire US Inc.’s INTUNIV® (guanfacine) extended-release tablet product. However, due to Shire US Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.3 Switching from Immediate-Release Guanfacine to Guanfacine Extended-Release
If switching from immediate-release guanfacine, discontinue that treatment, and titrate with guanfacine extended-release following above recommended schedule.
Do not substitute for immediate-release guanfacine tablets on a milligram-per-milligram basis, because of differing pharmacokinetic profiles. Guanfacine extended-release has significantly reduced C max (60% lower), bioavailability (43% lower), and a delayed T max (3 hours later) compared to those of the same dose of immediate-release guanfacine [see Clinical Pharmacology (12.3)].
2.4 Maintenance Treatment
Pharmacological treatment of ADHD may be needed for extended periods. Healthcare providers should periodically re-evaluate the long-term use of guanfacine extended-release, and adjust weight-based dosage as needed. The majority of children and adolescents reach optimal doses in the 0.05 to 0.12 mg/kg/day range.
Additional pediatric use information for patients ages 6 to 17 years is approved for Shire US Inc.’s INTUNIV® (guanfacine) extended-release tablet product. However, due to Shire US Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.5 Discontinuation of Treatment
Following discontinuation of guanfacine extended-release, patients may experience increases in blood pressure and heart rate [see Warnings and Precautions (5.4) and Adverse Reactions (6)]. Patients/caregivers should be instructed not to discontinue guanfacine extended-release without consulting their health care provider. Monitor blood pressure and pulse when reducing the dose or discontinuing the drug. Taper the daily dose in decrements of no more than 1 mg every 3 to 7 days to avoid rebound hypertension.
2.6 Missed Doses
When reinitiating patients to the previous maintenance dose after two or more missed consecutive doses, consider titration based on patient tolerability.
2.7 Dosage Adjustment with Concomitant Use of Strong and Moderate CYP3A4 Inhibitors or Inducers
Dosage adjustments for guanfacine extended-release are recommended with concomitant use of strong and moderate CYP3A4 inhibitors (e.g., ketoconazole), or CYP3A4 inducers (e.g., carbamazepine) (Table 2) [see Drug Interactions (7)].
Table 2: Guanfacine Extended-Release Dosage Adjustments for Patients Taking Concomitant CYP3A4 Inhibitors or Inducers Clinical Scenarios Starting guanfacine
extended-release
while currently on a
CYP3A4 modulatorContinuing
guanfacine extended-
release while adding a CYP3A4 modulatorContinuing guanfacine
extended-release while
stopping a CYP3A4
modulatorCYP3A4
Strong and Moderate InhibitorsDecrease guanfacine
extended-release
dosage to half the
recommended level.Decrease guanfacine
extended-release
dosage to half the
recommended level.Increase guanfacine
extended-release
dosage to recommended
level.CYP3A4
Strong and Moderate InducersConsider increasing
guanfacine extended-
release dosage up to
double the
recommended level.Consider
increasing guanfacine
extended-release
dosage up to double the recommended level over 1 to 2 weeks.Decrease guanfacine
extended-release
dosage to recommended
level over 1 to 2 weeks. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension, Bradycardia, and Syncope
Treatment with guanfacine extended-release can cause dose-dependent decreases in blood pressure and heart rate. Decreases were less pronounced over time of treatment. Orthostatic hypotension and syncope have been reported [see Adverse Reactions (6.1)].
Measure heart rate and blood pressure prior to initiation of therapy, following dose increases, and periodically while on therapy. Titrate guanfacine extended-release slowly in patients with a history of hypotension, and those with underlying conditions that may be worsened by hypotension and bradycardia; e.g., heart block, bradycardia, cardiovascular disease, vascular disease, cerebrovascular disease, or chronic renal failure. In patients who have a history of syncope or may have a condition that predisposes them to syncope, such as hypotension, orthostatic hypotension, bradycardia, or dehydration, advise patients to avoid becoming dehydrated or overheated. Monitor blood pressure and heart rate, and adjust dosages accordingly in patients treated concomitantly with antihypertensives or other drugs that can reduce blood pressure or heart rate or increase the risk of syncope.
5.2 Sedation and Somnolence
Somnolence and sedation were commonly reported adverse reactions in clinical studies [see Adverse Reactions (6.1)]. Before using guanfacine extended-release with other centrally active depressants, consider the potential for additive sedative effects. Caution patients against operating heavy equipment or driving until they know how they respond to treatment with guanfacine extended-release. Advise patients to avoid use with alcohol.
5.3 Cardiac Conduction Abnormalities
The sympatholytic action of guanfacine extended-release may worsen sinus node dysfunction and atrioventricular (AV) block, especially in patients taking other sympatholytic drugs. Titrate guanfacine extended-release slowly and monitor vital signs frequently in patients with cardiac conduction abnormalities or patients concomitantly treated with other sympatholytic drugs.
5.4 Rebound Hypertension
In post marketing experience, abrupt discontinuation of guanfacine extended-release tablets has resulted in clinically significant and persistent rebound hypertension above baseline levels and increases in heart rate. Hypertensive encephalopathy has also been reported in association with rebound hypertension with both guanfacine extended-release tablets and immediate release guanfacine [see Adverse Reactions (6.2)]. In these cases, high-dosage guanfacine was discontinued; concomitant stimulant use was also reported, which may potentially increase hypertensive response upon abrupt discontinuation of guanfacine. Children commonly have gastrointestinal illnesses that lead to vomiting, and a resulting inability to take medications, so they may be especially at risk for rebound hypertension.
To minimize the risk of rebound hypertension upon discontinuation, the total daily dose of guanfacine extended-release tablets should be tapered in decrements of no more than 1 mg every 3 to 7 days [see Dosage and Administration (2.5)]. Blood pressure and heart rate should be monitored when reducing the dose or discontinuing guanfacine extended-release tablets. If abrupt discontinuation occurs (especially with concomitant stimulant use), patients should be closely followed for rebound hypertension.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypotension, bradycardia, and syncope
[see Warnings and Precautions (5.1)]
- Sedation and somnolence
[see Warnings and Precautions (5.2)]
- Cardiac conduction abnormalities [see Warnings and Precautions (5.3)]
- Rebound Hypertension [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect clinical trial exposure to guanfacine extended-release in 2,028 patients. This includes 1,533 patients from completed studies in children and adolescents, ages 6 to 17 years and 495 patients in completed studies in adult healthy volunteers.
The mean duration of exposure of 446 patients that previously participated in two 2-year, open-label long-term studies was approximately 10 months.
Fixed Dose Trials
Table 3: Percentage of Patients Experiencing Most Common (≥5% and at least twice the rate for placebo) Adverse Reactions in Fixed Dose Studies 1 and 2 Guanfacine Extended-Release (mg) Adverse Reaction
TermPlacebo
(N=149)1 mg*
(N=61)2 mg
(N=150)3 mg
(N=151)4 mg
(N=151)All Doses of
Guanfacine Extended-Release (N=513)Somnolence a 11% 28% 30% 38% 51% 38% Fatigue 3% 10% 13% 17% 15% 14% Hypotension b 3% 8% 5% 7% 8% 7% Dizziness 4% 5% 3% 7% 10% 6% Lethargy 3% 2% 3% 8% 7% 6% Nausea 2% 7% 5% 5% 6% 6% Dry mouth 1% 0% 1% 6% 7% 4% *The lowest dose of 1 mg used in Study 2 was not randomized to patients weighing more than 50 kg.
aThe somnolence term includes somnolence, sedation, and hypersomnia.
bThe hypotension term includes hypotension, diastolic hypotension, orthostatic hypotension, blood pressure decreased, blood
pressure diastolic decreased, blood pressure systolic decreased).Table 4: Adverse Reactions Leading to Discontinuation (≥2% for all doses of Guanfacine Extended-Release and > rate in placebo) in Fixed Dose Studies 1 and 2 Guanfacine Extended-Release (mg) Adverse Reaction
TermPlacebo
(N=149)1 mg*
(N=61)2 mg
(N=150)3 mg
(N=151)4 mg
(N=151)All Doses of Guanfacine Extended-Release (N=513) n (%) n (%) n (%) n (%) n (%) n (%) Total patients 4 (3%) 2 (3%) 10 (7%) 15 (10%) 27 (18%) 54 (11%) Somnolence a 1 (1%) 2 (3%) 5 (3%) 6 (4%) 17 (11%) 30 (6%) Fatigue 0 (0%) 0 (0%) 2 (1%) 2 (1%) 4 (3%) 8 (2%) Adverse reactions leading to discontinuation in ≥ 2% in any dose group but did not meet this criteria in all doses combined: hypotension (hypotension, diastolic hypotension, orthostatic hypotension, blood pressure decreased, blood pressure diastolic decreased, blood pressure systolic decreased), headache, and dizziness.
*The lowest dose of 1 mg used in Study 2 was not randomized to patients weighing more than 50 kg.
aThe somnolence term includes somnolence, sedation, and hypersomnia.Table 5: Other Common Adverse Reactions (≥2%for all doses of Guanfacine Extended-Release and > rate than in placebo) in Fixed Dose Studies 1 and 2 Guanfacine Extended-Release (mg) Adverse Reaction Term Placebo
(N=149)1 mg*
(N=61)2 mg
(N=150)3 mg
(N=151)4 mg
(N=151)All Doses of Guanfacine Extended-Release
(N=513)Headache 19% 26% 25% 16% 28% 23% Abdominal Pain a 9% 10% 7% 11% 15% 11% Decreased Appetite 4% 5% 4% 9% 6% 6% Irritability 4% 5% 8% 3% 7% 6% Constipation 1% 2% 2% 3% 4% 3% Nightmare b 0% 0% 0% 3% 4% 2% Enuresis c 1% 0% 1% 3% 2% 2% Affect Lability d 1% 2% 1% 3% 1% 2% Adverse reactions ≥ 2% for all doses of guanfacine extended-release and > rate in placebo in any dose group but did not meet this criteria in all doses combined: insomnia (insomnia, initial insomnia, middle insomnia, terminal insomnia, sleep disorder), vomiting, diarrhea, abdominal/stomach discomfort (abdominal discomfort, epigastric discomfort, stomach discomfort), rash (rash, rash generalized, rash papular), dyspepsia, increased weight, bradycardia (bradycardia, sinus bradycardia), asthma (asthma, bronchospasm, wheezing), agitation, anxiety (anxiety, nervousness), sinus arrhythmia, blood pressure increased (blood pressure increased, blood pressure diastolic increased), and first degree atrioventricular block.
*The lowest dose of 1 mg used in Study 2 was not randomized to patients weighing more than 50 kg.
aThe abdominal pain term includes abdominal pain, abdominal pain lower, abdominal pain upper, and abdominal tenderness.
bThe nightmare term includes abnormal dreams, nightmare, and sleep terror.
cThe enuresis term includes enuresis, nocturia, and urinary incontinence.
dThe affect lability term includes affect lability and mood swings.Monotherapy Flexible Dose Trials
Table 6: Percentage of Patients Experiencing Most Common (≥5% and at least twice the rate for placebo) Adverse Reactions in the Monotherapy Flexible Dose Study 4 Guanfacine Extended-Release Adverse Reaction Term Placebo
(N=112)AM
(N=107)PM
(N=114)All Doses of
Guanfacine Extended-Release (N=221)Somnolence a 15% 57% 54% 56% Abdominal Pain b 7% 8% 19% 14% Fatigue 3% 10% 11% 11% Irritability 3% 7% 7% 7% Nausea 1% 6% 5% 5% Dizziness 3% 6% 4% 5% Vomiting 2% 7% 4% 5% Hypotension c 0% 6% 4% 5% Decreased Appetite 3% 6% 3% 4% Enuresis d 1% 2% 5% 4% aThe somnolence term includes somnolence, sedation, and hypersomnia.
bThe abdominal pain term includes abdominal pain, abdominal pain lower, abdominal pain upper, and abdominal tenderness
cThe hypotension term includes hypotension, diastolic hypotension, orthostatic hypotension, blood pressure decreased, blood pressure diastolic decreased, blood pressure systolic decreased).
dThe enuresis term includes enuresis, nocturia, and urinary incontinence.Table 7: Adverse Reactions Leading to Discontinuation (≥2% for all doses of Guanfacine Extended-Release and > rate than in placebo) in Monotherapy Flexible Dose Study 4 Guanfacine Extended-Release Adverse Reaction
TermPlacebo
(N=112)AM
(N=107)PM
(N=114)All Doses of Guanfacine Extended-Release
(N=221)n (%) n (%) n (%) n (%) Total patients 0 (0%) 8 (7%) 7 (6%) 15 (7%) Somnolence a 0 (0%) 4 (4%) 3 (3%) 7 (3%) Adverse reactions leading to discontinuation in greater than or equal to 2% in any dose group bud did not meet this criteria in all doses combined: fatigue
aThe somnolence term includes somnolence, sedation, and hypersomnia.Table 8: Other Common Adverse Reactions (≥2% for all doses of Guanfacine Extended-Release and > rate than in placebo) in the Monotherapy Flexible Dose Study 4 Guanfacine Extended-Release Adverse Reaction
TermPlacebo
(N=112)AM (N=107) PM (N=114) All Doses of Guanfacine Extended-Release
(N=221)Headache 11% 18% 16% 17% Insomnia a 6% 8% 6% 7% Diarrhea 4% 4% 6% 5% Lethargy 0% 4% 3% 3% Constipation 2% 2% 4% 3% Dry Mouth 1% 3% 3% 3% Adverse reactions ≥ 2% for all doses of guanfacine extended-release and > rate in placebo in any dose group but did not meet this criteria in all doses combined: affect lability (affect lability, mood swings), increased weight, syncope/loss of consciousness (loss of consciousness, presyncope, syncope), dyspepsia, tachycardia (tachycardia, sinus tachycardia), and bradycardia (bradycardia, sinus bradycardia).
aThe insomnia term includes insomnia, initial insomnia, middle insomnia, terminal insomnia, and sleep disorder.Adjunctive Trial
Table 11: Percentage of Patients Experiencing Most Common (≥5% and at least twice the rate for placebo) Adverse Reactions in the Short-Term Adjunctive Study 3 Guanfacine Extended-Release + stimulant Adverse Reaction Term Placebo+ stimulant (N=153) AM
(N=150)PM
(N=152)All Doses (N=302) Somnolence a 7% 18% 18% 18% Insomnia b 6% 10% 14% 12% Abdominal Pain c 3% 8% 12% 10% Fatigue 3% 12% 7% 10% Dizziness 4% 10% 5% 8% Decreased Appetite 4% 7% 8% 7% Nausea 3% 3% 7% 5% aThe somnolence term includes somnolence, sedation, and hypersomnia.
bThe insomnia term includes insomnia, initial insomnia, middle insomnia, terminal insomnia, and sleep disorder.
cThe abdominal pain term includes abdominal pain, abdominal pain lower, abdominal pain upper, and abdominal tenderness.There were no specific adverse reactions greater than or equal to 2% in any treatment group that led to discontinuation in the short-term adjunctive study (Study 3).
Table 12: Other Common Adverse Reactions (≥2% for all doses of Guanfacine Extended-Release and > rate than in placebo) in the Short-Term Adjunctive Study 3 Guanfacine Extended-Release + stimulant Adverse Reaction Term Placebo
(N=153)AM
(N=150)PM
(N=152)All Doses of Guanfacine Extended-Release
(N=302)Headache 13% 21% 21% 21% Diarrhea 1% 4% 3% 4% Hypotension a 0% 4% 2% 3% Constipation 0% 2% 3% 2% Affect Lability b 1% 3% 2% 2% Dry Mouth 0% 1% 3% 2% Bradycardia c 0% 1% 3% 2% Postural Dizziness 0% 1% 3% 2% Rash d 1% 1% 2% 2% Nightmare e 1% 2% 1% 2% Tachycardia f 1% 2% 1% 2% Adverse reactions greater than or equal to 2% for all doses of guanfacine extended-release and greater than rate in placebo in any dose group but did not meet this criteria in all doses combined: irritability, vomiting, asthma (asthma, bronchospasm, wheezing), and enuresis (enuresis, nocturia, urinary incontinence).
aThe hypotension term includes hypotension, diastolic hypotension, orthostatic hypotension, blood pressure decreased, blood pressure diastolic decreased, blood pressure systolic decreased.
bThe affect lability term includes affect lability and mood swings.
cThe bradycardia term includes bradycardia and sinus bradycardia.
dThe rash term includes rash, rash generalized, and rash papular.
eThe nightmare term includes abnormal dreams, nightmare, and sleep terror.
fThe tachycardia term includes tachycardia and sinus tachycardia.Effects on Blood Pressure and Heart Rate
In the monotherapy pediatric, short-term, controlled trials (Studies 1 and 2), the maximum mean changes from baseline in seated systolic blood pressure, diastolic blood pressure, and pulse were -5.4 mmHg, -3.4 mmHg, and -5.5 bpm, respectively, for all doses combined (generally one week after reaching target doses). For the respective fixed doses 1 mg/day, 2 mg/day, 3 mg/day or 4 mg/day the maximum mean changes in seated systolic blood pressure were -4.3 mmHg, -5.5 mmHg, -5.4 mmHg and -8.2 mmHg. For these respective fixed doses the maximum mean changes in seated diastolic blood pressure were -3.4 mmHg, -3.3 mmHg, -4.4 mmHg and -5.4 mmHg. For these respective fixed doses the maximum mean changes in seated pulse were -4.8 bpm, -3.1 bpm, -6.5 bpm and -8.6 bpm. Decreases in blood pressure and heart rate were usually modest and asymptomatic; however, hypotension and bradycardia can occur. Hypotension was reported as an adverse reaction for 7% of the guanfacine extended-release group and 3% of the placebo group. This includes orthostatic hypotension, which was reported for 1% of the guanfacine extended-release group and none in the placebo group. These findings were generally similar in the monotherapy flexible dose trial (Study 4). In the adjunctive trial, hypotension (3%) and bradycardia (2%) were observed in patients treated with guanfacine extended-release as compared to none in the placebo group. In long-term, open-label studies, (mean exposure of approximately 10 months), maximum decreases in systolic and diastolic blood pressure occurred in the first month of therapy. Decreases were less pronounced over time. Syncope occurred in 1% of pediatric patients in the clinical program. The majority of these cases occurred in the long-term, open-label studies.
Effects on Height, Weight, and Body Mass Index (BMI)
Patients taking guanfacine extended-release demonstrated similar growth compared to normative data. Patients taking guanfacine extended-release had a mean increase in weight of 0.5 kg compared to those receiving placebo over a comparable treatment period. Patients receiving guanfacine extended-release for at least 12 months in open-label studies gained an average of 8 kg in weight and 8 cm (3 in) in height. The height, weight, and BMI percentile remained stable in patients at 12 months in the long-term studies compared to when they began receiving guanfacine extended-release.
Other Adverse Reactions Observed in Clinical Studies
Table 13 includes additional adverse reactions observed in short-term, placebo-controlled and long-term, open-label clinical studies not included elsewhere in section 6.1, listed by organ system.
Table 13: Other Adverse Reactions Observed in Clinical Studies Body System Adverse Reaction Cardiac Atrioventricular block General Asthenia, chest pain Immune System Disorders Hypersensitivity Investigations Increased alanine amino transferase Nervous system Convulsion Renal Increased urinary frequency Vascular Hypertension, pallor Additional pediatric use information for patients ages 6 to 17 years is approved for Shire US Inc.’s INTUNIV® (guanfacine) extended-release tablet product. However, due to Shire US Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of guanfacine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Less frequent, possibly guanfacine-related events observed in the postmarketing study and/or reported spontaneously, not included in section 6.1, include:
General: edema, malaise, tremor
Cardiovascular: palpitations, tachycardia, rebound hypertension, hypertensive encephalopathy
Central Nervous System: paresthesias, vertigo
Eye Disorders: blurred vision
Musculoskeletal System: arthralgia, leg cramps, leg pain, myalgia
Psychiatric: confusion, hallucinations
Reproductive System, Male: impotence
Respiratory System: dyspnea
Skin and Appendages: alopecia, dermatitis, exfoliative dermatitis, pruritus, rash
Special Senses: alterations in taste - Hypotension, bradycardia, and syncope
[see Warnings and Precautions (5.1)]
-
7 DRUG INTERACTIONS
Table 14 contains clinically important drug interactions with guanfacine extended-release [see Clinical Pharmacology (12.3)].
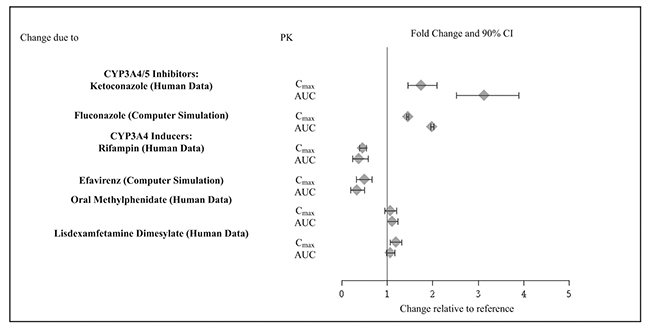
Table 14: Clinically Important Drug Interactions: Effect of other Drugs on Guanfacine Extended-Release Concomitant Drug Name or
Drug ClassClinical Rationale and Magnitude of Drug Interaction Clinical Recommendation Strong and moderate CYP3A4 inhibitors, e.g., ketoconazole, fluconazole Guanfacine is primarily metabolized by CYP3A4 and its plasma concentrations can be significantly affected
resulting in an increase in exposureConsider dose reduction [see Dosage and administration (2.7)] Strong and moderate CYP3A4 inducers, e.g., rifampin, efavirenz Guanfacine is primarily metabolized by CYP3A4 and its
plasma concentrations can be significantly affected resulting in a decrease in exposureConsider dose increase
[see Dosage and administration (2.7)] -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Risk Summary
There are no adequate and well-controlled studies of guanfacine extended-release in pregnant women. No fetal harm was observed in rats and rabbits with administration of guanfacine at 4 and 2.7 times, respectively, the maximum recommended human dose. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Animal data
Reproduction studies conducted in rats have shown that guanfacine crosses the placenta. However, administration of guanfacine to rats and rabbits at 4 and 2.7 times, respectively, the maximum recommended human dose of 0.12 mg/kg/day on a mg/m 2 basis resulted in no evidence of harm to the fetus. Higher doses (13.5 times the maximum recommended human dose in both rabbits and rats) were associated with reduced fetal survival and maternal toxicity.
8.3 Nursing Mothers
It is not known whether guanfacine is excreted in human milk; however, guanfacine is excreted in rat milk. Because many drugs are excreted in human milk, caution should be exercised when guanfacine extended-release is administered to a nursing woman. Observe human milk-fed infants for sedation and somnolence.
8.4 Pediatric Use
Safety and efficacy of guanfacine extended-release in pediatric patients less than 6 years of age have not been established. The efficacy of guanfacine extended-release was studied for the treatment of ADHD in three controlled monotherapy clinical trials (up to 8 weeks in duration), and one controlled adjunctive trial with psychostimulants (8 weeks in duration) in children and adolescents ages 6 to 17 who met DSM-IV ® criteria for ADHD [see Adverse Reactions (6) and Clinical Studies (14)].
Additional pediatric use information for patients ages 6 to 17 years is approved for Shire US Inc.’s INTUNIV® (guanfacine) extended-release tablet product. However, due to Shire US Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Animal Data
In studies in juvenile rats, guanfacine alone produced a slight delay in sexual maturation in males and females at 2 to 3 times the maximum recommended human dose (MRHD). Guanfacine in combination with methylphenidate produced a slight delay in sexual maturation and decreased growth as measured by a decrease in bone length in males at a dose of guanfacine comparable to the MRHD and a dose of methylphenidate approximately 4 times the MRHD.
In a study where juvenile rats were treated with guanfacine alone from 7 to 59 days of age, development was delayed as indicated by a slight delay in sexual maturation and decreased body weight gain in males at 2 mg/kg/day and in females at 3 mg/kg/day. The No Adverse Effect Level (NOAEL) for delayed sexual maturation was 1 mg/kg/day, which is equivalent to the MRHD of 4 mg/day, on a mg/m 2 basis. The effects on fertility were not evaluated in this study.
In a study where juvenile rats were treated with guanfacine in combination with methylphenidate from 7 to 59 days of age, a decrease in ulna bone length and a slight delay in sexual maturation were observed in males given 1 mg/kg/day of guanfacine in combination with 50 mg/kg/day of methylphenidate. The NOAELs for these findings were 0.3 mg/kg of guanfacine in combination with 16 mg/kg/day of methylphenidate, which are equivalent to 0.3 and 1.4 times the MRHD of 4 mg/day and 54 mg/day for guanfacine and methylphenidate, respectively, on a mg/m 2 basis. These findings were not observed with guanfacine alone at 1 mg/kg/day or methylphenidate alone at 50 mg/kg/day.
8.5 Geriatric Use
The safety and efficacy of guanfacine extended-release in geriatric patients have not been established.
8.6 Renal Impairment
It may be necessary to reduce the dosage in patients with significant impairment of renal function [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
It may be necessary to reduce the dosage in patients with significant impairment of hepatic function [see Clinical Pharmacology (12.3)].
- 9 DRUG ABUSE AND DEPENDENCE
-
10 OVERDOSAGE
Symptoms
Postmarketing reports of guanfacine overdosage indicate that hypotension, drowsiness, lethargy, and bradycardia have been observed following overdose. Initial hypertension may develop early and may be followed by hypotension. Similar symptoms have been described in voluntary reports to the American Association of Poison Control Center’s National Poison Data System. Miosis of the pupils may be noted on examination. No fatal overdoses of guanfacine have been reported in published literature.
Treatment
Consult a Certified Poison Control Center by calling 1-800-222-1222 for up-to-date guidance and advice.
Management of guanfacine extended-release overdose should include monitoring for and the treatment of initial hypertension, if that occurs, as well as hypotension, bradycardia, lethargy and respiratory depression. Children and adolescents who develop lethargy should be observed for the development of more serious toxicity including coma, bradycardia and hypotension for up to 24 hours, due to the possibility of delayed onset hypotension.
-
11 DESCRIPTION
Guanfacine extended-release is a once-daily, extended-release formulation of guanfacine hydrochloride (HCl), in a matrix tablet formulation for oral administration only. The chemical designation is N-amidino-2-(2, 6-dichlorophenyl) acetamide monohydrochloride.
The chemical structure is:
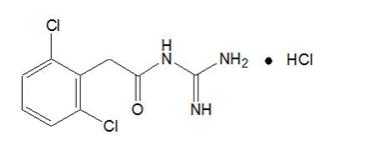
C 9H 9Cl 2N 3O•HCl M.W. 282.55
Guanfacine hydrochloride, USP is a white to off-white crystalline powder, sparingly soluble in water (approximately 1 mg/mL) and alcohol and slightly soluble in acetone. The only organic solvent in which it has relatively high solubility is methanol (greater than 30 mg/mL). Each tablet contains guanfacine hydrochloride, USP equivalent to 1 mg, 2 mg, 3 mg, or 4 mg of guanfacine base. The tablets also contain colloidal silicon dioxide, crospovidone, fumaric acid, glyceryl behenate, hydroxypropyl cellulose, hypromellose, lactose monohydrate, microcrystalline cellulose, and povidone. In addition, the 1 mg and 2 mg tablets contain FD&C Yellow #6 Aluminum Lake and the 3 mg and 4 mg tablets contain D&C Yellow #10 Aluminum Lake.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Guanfacine is a central alpha 2A-adrenergic receptor agonist. Guanfacine is not a central nervous system (CNS) stimulant. The mechanism of action of guanfacine in ADHD is not known.
12.2 Pharmacodynamics
Guanfacine is a selective central alpha 2A-adrenergic receptor agonist in that it has a 15 to 20 times higher affinity for this receptor subtype than for the alpha 2B or alpha 2C subtypes.
Guanfacine is a known antihypertensive agent. By stimulating central alpha 2A-adrenergic receptors, guanfacine reduces sympathetic nerve impulses from the vasomotor center to the heart and blood vessels. This results in a decrease in peripheral vascular resistance and a reduction in heart rate.
In a thorough QT study, the administration of two dose levels of immediate-release guanfacine (4 mg and 8 mg) produced concentrations approximately 2 to 4 times the concentrations observed with the maximum recommended dose of guanfacine extended-release of 0.12 mg/kg. Guanfacine was not shown to prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption and Distribution
Guanfacine is readily absorbed and approximately 70% bound to plasma proteins independent of drug concentration. After oral administration of guanfacine extended-release the time to peak plasma concentration is approximately 5 hours in children and adolescents with ADHD.
Immediate-release guanfacine and guanfacine extended-release have different pharmacokinetic characteristics; dose substitution on a milligram per milligram basis will result in differences in exposure.
A comparison across studies suggests that the C max is 60% lower and AUC 0-∞ 43% lower, respectively, for guanfacine extended-release compared to immediate-release guanfacine. Therefore, the relative bioavailability of guanfacine extended-release to immediate-release guanfacine is 58%. The mean pharmacokinetic parameters in adults following the administration of guanfacine extended-release 1 mg once daily and immediate-release guanfacine 1 mg once daily are summarized in Table 15.
Table 15: Comparison of Pharmacokinetics: Guanfacine Extended-Release vs. Immediate release Guanfacine in Adults Parameter Guanfacine
Extended-Release
1 mg once daily
(n=52)Immediate-release
guanfacine
1 mg once daily
(n=12)C max (ng/mL) 1.0 ± 0.3 2.5 ± 0.6 AUC 0-∞ (ng.h/mL) 32 ± 9 56 ± 15 t max (h) 6.0 (4.0 – 8.0) 3.0 (1.5-4.0) t ½ (h) 18 ± 4 16 ± 3 Note: Values are mean +/- SD, except for t max which is median (range)
Figure 1: Comparison of Pharmacokinetics: Guanfacine Extended-Release vs. Immediate-release Guanfacine in Adults
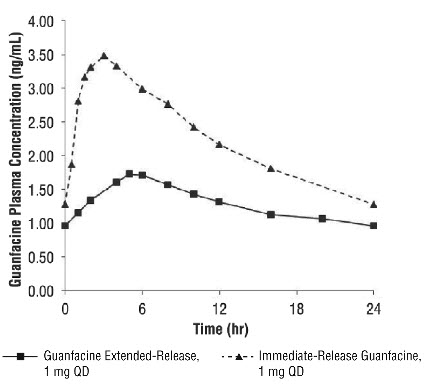
Exposure to guanfacine was higher in children (ages 6 to 12) compared to adolescents (ages 13 to 17) and adults. After oral administration of multiple doses of guanfacine extended-release 4 mg, the C max was 10 ng/mL compared to 7 ng/mL and the AUC was 162 ng•h/mL compared to 116 ng•h/mL in children (ages 6 to 12) and adolescents (ages 13 to 17), respectively. These differences are probably attributable to the lower body weight of children compared to adolescents and adults.
The pharmacokinetics were affected by intake of food when a single dose of guanfacine extended-release 4 mg was administered with a high-fat breakfast. The mean exposure increased (C max ~75% and AUC ~40%) compared to dosing in a fasted state.
Dose Proportionality
Following administration of guanfacine extended-release in single doses of 1 mg, 2 mg, 3 mg, and 4 mg to adults, C max and AUC 0-∞ of guanfacine were proportional to dose.
Metabolism and Elimination
In vitro studies with human liver microsomes and recombinant CYP’s demonstrated that guanfacine was primarily metabolized by CYP3A4. In pooled human hepatic microsomes, guanfacine did not inhibit the activities of the major cytochrome P450 isoenzymes (CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A4/5). Guanfacine is a substrate of CYP3A4/5 and exposure is affected by CYP3A4/5 inducers/inhibitors.
Studies in Specific Populations
Renal Impairment
The impact of renal impairment on the pharmacokinetics of guanfacine in children was not assessed. In adult patients with impaired renal function, the cumulative urinary excretion of guanfacine and the renal clearance diminished as renal function decreased. In patients on hemodialysis, the dialysis clearance was about 15% of the total clearance. The low dialysis clearance suggests that the hepatic elimination (metabolism) increases as renal function decreases.
Hepatic Impairment
The impact of hepatic impairment on PK of guanfacine in children was not assessed. Guanfacine in adults is cleared both by the liver and the kidney, and approximately 50% of the clearance of guanfacine is hepatic [see Hepatic Impairment (8.7)].
Drug Interaction Studies
Guanfacine is primarily metabolized by CYP3A4 and its plasma concentrations can be affected significantly by CYP3A4 inhibitors or inducers (Figure 2).
Guanfacine does not significantly affect exposures of methylphenidate and lisdexamfetamine when coadministered (Figure 3).
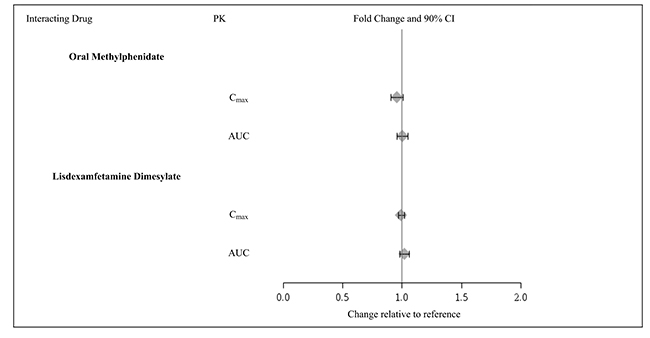
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenic effect of guanfacine was observed in studies of 78 weeks in mice or 102 weeks in rats at doses up to 6.8 times the maximum recommended human dose of 0.12 mg/kg/day on a mg/m 2 basis.
Mutagenesis
Guanfacine was not genotoxic in a variety of test models, including the Ames test and an in vitro chromosomal aberration test; however, a marginal increase in numerical aberrations (polyploidy) was observed in the latter study.
Impairment of Fertility
No adverse effects were observed in fertility studies in male and female rats at doses up to 22 times the maximum recommended human dose on a mg/m 2 basis.
-
14 CLINICAL STUDIES
Efficacy of guanfacine extended-release in the treatment of ADHD was established in children and adolescents (6 to 17 years) in:
- Three short-term, placebo-controlled monotherapy trials (Studies 1, 2, and 4)
- One short-term, placebo-controlled adjunctive trial with psychostimulants (Study 3).
Studies 1 and 2: Fixed-dose Guanfacine Extended-Release Monotherapy
Study 1 (301 study) was a double-blind, placebo-controlled, parallel-group, fixed-dose study, in which efficacy of once daily dosing with guanfacine extended-release (2 mg, 3 mg and 4 mg) was evaluated for 5 weeks (n=345) in children and adolescents aged 6 to 17 years. Study 2 (304 study) was a double-blind, placebo-controlled, parallel-group, fixed-dose study, in which efficacy of once daily dosing with guanfacine extended-release (1 mg, 2 mg, 3 mg and 4 mg) was evaluated for 6 weeks (n=324) in children and adolescents aged 6 to 17 years. In both studies, randomized patients in 2 mg, 3 mg and 4 mg dose groups were titrated to their target fixed dose, and continued on the same dose until a dose tapering phase started. The lowest dose of 1 mg used in Study 2 was not randomized to patients weighing more than 50 kg. Patients who weighed less than 25 kg were not included in either study.
Signs and symptoms of ADHD were evaluated on a once weekly basis using the clinician administered and scored ADHD Rating Scale (ADHD-RS-IV), which includes both hyperactive/impulsive and inattentive subscales. The primary efficacy outcome was the change from baseline to endpoint in ADHD-RS-IV total scores. Endpoint was defined as the last post-randomization treatment week for which a valid score was obtained prior to dose tapering (up to Week 5 in Study 1 and up to Week 6 in Study 2).
The mean reductions in ADHD-RS-IV total scores at endpoint were statistically significantly greater for guanfacine extended-release compared to placebo for Studies 1 and 2. Placebo-adjusted changes from baseline were statistically significant for each of the 2 mg, 3 mg, and 4 mg guanfacine extended-release randomized treatment groups in both studies, as well as the 1 mg guanfacine extended-release treatment group that was included only in Study 2 (see Table 16).
Dose-responsive efficacy was evident, particularly when data were examined on a weight-adjusted (mg/kg) basis. When evaluated over the dose range of 0.01 to 0.17 mg/kg/day, clinically relevant improvements were observed beginning at doses in the range 0.05 to 0.08 mg/kg/day. Doses up to 0.12 mg/kg/day were shown to provide additional benefit.
In the monotherapy trials (Studies 1 and 2), subgroup analyses were performed to identify any differences in response based on gender or age (6 to 12 vs. 13 to 17). Analyses of the primary outcome did not suggest any differential responsiveness on the basis of gender. Analyses by age revealed a statistically significant treatment effect only in the 6 to 12 age subgroup. Due to the relatively small proportion of adolescent patients (ages 13 to 17) enrolled into these studies (approximately 25%), these data may not have been sufficient to demonstrate efficacy in the adolescent patients. In these studies, patients were randomized to a fixed dose of guanfacine extended-release rather than optimized by body weight. Therefore, some adolescent patients were randomized to a dose that might have resulted in relatively lower plasma guanfacine concentrations compared to the younger patients. Over half (55%) of the adolescent patients received doses of 0.01 to 0.04 mg/kg. In studies in which systematic pharmacokinetic data were obtained, there was a strong inverse correlation between body weight and plasma guanfacine concentrations.
Table 16: Fixed Dose Studies Study Primary Efficacy Measure: ADHD-RS-IV Total Score Number Mean Baseline LS Mean Change Placebo-subtracted (Age Range) Treatment Group Score (SD) from Baseline (SE) Differencea (95% CI) Study 1 Guanfacine Extended-Release 2 mg * 36.1 (9.99) -15.9 (1.37) -7.4 (-11.3, -3.5) (6 to 17 years) Guanfacine Extended-Release 3 mg * 36.8 (8.72) -16.0 (1.38) -7.5 (-11.4, -3.6) Guanfacine Extended-Release 4 mg * 38.4 (9.21) -18.5 (1.39) -10.0 (-13.9, -6.1) Placebo 38.1 (9.34) -8.5 (1.42) -- Study 2 Guanfacine Extended-Release 1 mg *^ 41.7 (7.81) -19.4 (1.69) -6.8 (-11.3, -2.2) (6 to 17 years) Guanfacine Extended-Release 2 mg * 39.9 (8.74) -18.1 (1.60) -5.4 (-9.9, -0.9) Guanfacine Extended-Release 3 mg * 39.1 (9.22) -20.0 (1.64) -7.3 (-11.8, -2.8) Guanfacine Extended-Release 4 mg * 40.6 (8.57) -20.6 (1.60) -7.9 (-12.3, -3.4) Placebo 39.3 (8.85) -12.7 (1.60) -- SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
a Difference (drug minus placebo) in least-squares mean change from baseline.
* Doses statistically significantly superior to placebo.
^ The lowest dose of 1 mg used in Study 2 was not randomized to patients weighing more than 50 kg.Study 3: Flexible-dose Guanfacine Extended-Release as Adjunctive Therapy to Psychostimulants
Study 3 (313 study) was a double-blind, randomized, placebo-controlled, dose-optimization study, in which efficacy of once daily optimized dosing (morning or evening) with guanfacine extended-release (1 mg, 2 mg, 3 mg and 4 mg), when coadministered with psychostimulants, was evaluated for 8 weeks, in children and adolescents aged 6 to 17 years with a diagnosis of ADHD, with a sub-optimal response to stimulants (n=455). Patients were started at the 1 mg guanfacine extended-release dose level and were titrated weekly over a 5-week dose-optimization period to an optimal guanfacine extended-release dose not to exceed 4 mg/day based on tolerability and clinical response. The dose was then maintained for a 3-week dose maintenance period before entry to 1 week of dose tapering. Patients took guanfacine extended-release either in the morning or the evening while maintaining their current dose of psychostimulant treatment given each morning. Allowable psychostimulants in the study were ADDERALL XR ®, VYVANSE ®, CONCERTA ®, FOCALIN XR ®, RITALIN LA ®, METADATE CD ® or FDA-approved generic equivalents.
Symptoms of ADHD were evaluated on a weekly basis by clinicians using the ADHD Rating Scale (ADHD-RS-IV), which includes both hyperactive/impulsive and inattentive subscales. The primary efficacy outcome was the change from baseline to endpoint in ADHD-RS-IV total scores. Endpoint was defined as the last post- randomization treatment week prior to dose tapering for which a valid score was obtained (up to Week 8).
Mean reductions in ADHD-RS-IV total scores at endpoint were statistically significantly greater for guanfacine extended-release given in combination with a psychostimulant compared to placebo given with a psychostimulant for Study 3, for both morning and evening guanfacine extended-release dosing (see Table 17). Nearly two-thirds (64.2%) of patients reached optimal doses in the 0.05 to 0.12 mg/kg/day range.
Study 4: Flexible-dose Guanfacine Extended-Release Monotherapy
Study 4 (314 study) was a double-blind, randomized, placebo-controlled, dose-optimization study, in which efficacy of once daily dosing (morning or evening) with guanfacine extended-release (1 mg, 2 mg, 3 mg, and 4 mg) was evaluated for 8 weeks in children aged 6 to 12 years (n=340).
Signs and symptoms of ADHD were evaluated on a once weekly basis using the clinician administered and scored ADHD Rating Scale (ADHD-RS-IV), which includes both hyperactive/impulsive and inattentive subscales. The primary efficacy outcome was the change from baseline score at endpoint on the ADHD-RS-IV total scores. Endpoint was defined as the last post-randomization treatment week for which a valid score was obtained prior to dose tapering (up to Week 8).
Mean reductions in ADHD-RS-IV total scores at endpoint were statistically significantly greater for guanfacine extended-release compared to placebo in both AM and PM dosing groups of guanfacine extended-release (see Table 17).
Table 17: Flexible-Dose Studies Primary Efficacy Measure: ADHD-RS-IV Total Study Mean Baseline LS Mean Placebo- Number Score (SD) Change from subtracted (Age Range) Treatment Group Baseline (SE) Differenceb (95% CI) Study 3 a Guanfacine Extended-Release 1 to 4 mg AM* 37.6 (8.13) -20.3 (0.97) -4.5 (-7.5, -1.4) (6 to 17 years) Guanfacine Extended-Release 1 to 4 mg PM* 37.0 (7.65) -21.2 (0.97) -5.3 (-8.3, -2.3) Placebo 37.7 (7.75) -15.9 (0.96) -- Study 4 Guanfacine Extended-Release 1 to 4 mg AM* 41.7 (6.39) -20.0 (1.23) -9.4 (-12.8, -6.0) (6 to 12 years) Guanfacine Extended-Release 1 to 4 mg PM* 41.6 (6.66) -20.4 (1.19) -9.8 (-13.1, -6.4) Placebo 42.9 (6.29) -10.6 (1.20) -- SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
a Treatment was given in combination with a psychostimulant.
b Difference (drug minus placebo) in least-squares mean change from baseline.
* Doses statistically significantly superior to placebo.Additional pediatric use information for patients ages 6 to 17 years is approved for Shire US Inc.’s INTUNIV® (guanfacine) extended-release tablet product. However, due to Shire US Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
- Three short-term, placebo-controlled monotherapy trials (Studies 1, 2, and 4)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Guanfacine extended-release tablets are available as follows:
1 mg - Each orange, round tablet debossed with
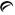 on one side and 850 on the other side contains guanfacine hydrochloride, USP equivalent to 1 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-324-01).
on one side and 850 on the other side contains guanfacine hydrochloride, USP equivalent to 1 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-324-01).
2 mg - Each orange, oval tablet debossed with
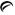 on one side and 851 on the other side contains guanfacine hydrochloride, USP equivalent to 2 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-325-01).
on one side and 851 on the other side contains guanfacine hydrochloride, USP equivalent to 2 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-325-01).
3 mg - Each yellow, round tablet debossed with
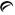 on one side and 853 on the other side contains guanfacine hydrochloride, USP equivalent to 3 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-326-01).
on one side and 853 on the other side contains guanfacine hydrochloride, USP equivalent to 3 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-326-01).
4 mg - Each yellow, oval tablet debossed with
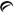 on one side and 855 on the other side contains guanfacine hydrochloride, USP equivalent to 4 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-327-01).
on one side and 855 on the other side contains guanfacine hydrochloride, USP equivalent to 4 mg of guanfacine base. Tablets are supplied in bottles of 100 (NDC 42291-327-01).
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Dosing and Administration
Instruct patients to swallow guanfacine extended-release tablets whole with water, milk or other liquid. Tablets should not be crushed, chewed or broken prior to administration because this may increase the rate of release of the active drug. Patients should not take guanfacine extended-release tablets together with a high-fat meal, since this can raise blood levels of guanfacine extended-release. Instruct the parent or caregiver to supervise the child or adolescent taking guanfacine extended-release tablets and to keep the bottle of tablets out of reach of children.
Advise patients not to abruptly discontinue guanfacine extended-release tablets as abrupt discontinuation can result in clinically significant rebound hypertension. Concomitant stimulant use and abrupt discontinuation of guanfacine extended-release tablets may increase this hypertensive response. Instruct patients on how to properly taper the dose to minimize the risk of rebound hypertension [see Dosage and Administration (2.5) and Warnings and Precautions (5.4)].
Adverse Reactions
Advise patients that sedation can occur, particularly early in treatment or with dose increases. Caution against operating heavy equipment or driving until they know how they respond to treatment with guanfacine extended-release tablets [see Warnings and Precautions (5.2)]. Headache and abdominal pain can also occur. If any of these symptoms persist, or other symptoms occur, the patient should be advised to discuss the symptoms with the health care provider.
Advise patients to avoid becoming dehydrated or overheated, which may potentially increase the risks of hypotension and syncope [see Warnings and Precautions (5.1)]. Advise patients to avoid use with alcohol.
Brands listed are the trademarks of their respective owners.
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
Mfg. Rev. 01/18
AV Rev. 10/18 (P) -
Patient Information
Guanfacine (gwahn' fa seen)
Extended-Release Tablets
Rx Only
Read the Patient Information that comes with guanfacine extended-release before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What areguanfacine extended-release tablets?
Guanfacine extended-release tablets are a prescription medicine used to treat the symptoms of attention deficit hyperactivity disorder (ADHD). Guanfacine extended-release tablets may be used alone or with ADHD stimulant medicines.
Guanfacine extended-release is not a central nervous system (CNS) stimulant.
It is not known if guanfacine extended-release tablets are safe and effective in children younger than 6 years of age.
Who should not take guanfacine extended-release tablets?
Do not take guanfacine extended-release tablets if you are allergic to guanfacine or any of the ingredients in guanfacine extended-release tablets. See the end of this leaflet for a complete list of ingredients in guanfacine extended-release tablets.
What should I tell my doctor before taking guanfacine extended-release tablets?
Before you take guanfacine extended-release tablets, tell your doctor if you:
- have heart problems or a low heart rate
- have fainted
- have low or high blood pressure
- have liver or kidney problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if guanfacine extended-release tablets will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if guanfacine passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking guanfacine extended-release.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Guanfacine extended-release tablets may affect the way other medicines work, and other medicines may affect how guanfacine extended-release tablets work.
Especially tell your doctor if you take:
- ketoconazole
- medicines that can affect enzyme metabolism
- high blood pressure medicine
- sedatives
- benzodiazepines
- barbiturates
- antipsychotics
Ask your doctor or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I takeguanfacine extended-release tablets?
- Take guanfacine extended-release tablets exactly as your doctor tells you.
- Your doctor may change your dose. Do not change your dose of guanfacine extended-release tablets without talking to your doctor.
- Try not to miss your dose of guanfacine extended-release tablets. If you miss a dose of guanfacine extended-release tablets, take the next dose at your regular time. If you miss 2 or more doses, talk to your doctor, as you may need to restart guanfacine extended-release tablets with a lower dose.
- Do not take a double dose to make up for a missed dose.
- Do not stop taking guanfacine extended-release tablets without talking to your doctor.
- Guanfacine extended-release tablets should be taken 1 time a day in the morning or in the evening, either alone or in combination with an ADHD stimulant medicine that your doctor may prescribe. Your doctor will tell you when to take guanfacine extended-release tablets and when to take your ADHD stimulant medication.
- Guanfacine extended-release tablets should be swallowed whole with a small amount of water, milk, or other liquid.
- Do not crush, chew, or break guanfacine extended-release tablets. Tell your doctor if you cannot swallow guanfacine extended-release tablets whole.
- Do not take guanfacine extended-release tablets with a high-fat meal.
- Your doctor will check your blood pressure and heart rate while you take guanfacine extended-release tablets.
- If you take too many guanfacine extended-release tablets, call your local Poison Control Center at
1-800-222-1222 or go to the nearest emergency room right away.
What should I avoid while taking guanfacine extended-release tablets?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how guanfacine extended-release tablets affect you. Guanfacine extended-release tablets can slow your thinking and motor skills.
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking guanfacine extended-release tablets until you talk with your doctor. Guanfacine extended-release tablets taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not become dehydrated or overheated. This may increase your chance of having low blood pressure or fainting while taking guanfacine extended-release tablets.
- Do not suddenly stop guanfacine extended-release tablets. Tell your healthcare provider if you have been vomiting and cannot take guanfacine extended-release tablets, you may be at risk for rebound hypertension.
What are the possible side effects of guanfacine extended-release tablets?
Guanfacine extended-release tabletsmay cause serious side effects including:
- low blood pressure
- low heart rate
- fainting
- sleepiness
- increased blood pressure and heart rate after suddenly stopping guanfacine extended-release tablets (rebound hypertension). Suddenly stopping guanfacine extended-release tablets can cause increased blood pressure and heart rate and other withdrawal symptoms such as headache, confusion, nervousness, agitation, and tremors. If these symptoms continue to get worse and are left untreated, it could lead to a very serious condition including very high blood pressure, feeling very sleepy or tired, severe headache, vomiting, vision problems, seizures.
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of guanfacine extended-release tablets include:
- sleepiness
- tiredness
- trouble sleeping
- low blood pressure
- nausea
- stomach pain
- dizziness
- dry mouth
- irritability
- vomiting
- slow heart rate
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of guanfacine extended-release tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at
1-800-FDA-1088.How should I storeguanfacine extended-release tablets?
- Store guanfacine extended-release tablets between 68°F to 77°F (20°C to 25°C)
Keep guanfacine extended-release tablets and all medicines out of the reach of children.
General Information about the safe and effective use guanfacine extended-release tablets
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use guanfacine extended-release tablets for a condition for which they were not prescribed. Do not give guanfacine extended-release tablets to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about guanfacine extended-release tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about guanfacine extended-release tablets that is written for health professionals.
For more information, call AvKARE, Inc. at 1-855-361-3993.
What are the ingredients in guanfacine extended-release tablets?
Active ingredient: guanfacine hydrochloride
Inactive ingredients: colloidal silicon dioxide, crospovidone, fumaric acid, glyceryl behenate, hydroxypropyl cellulose, hypromellose, lactose monohydrate, microcrystalline cellulose, and povidone. In addition, the 1 mg and 2 mg tablets contain FD&C Yellow #6 Aluminum Lake and the 3 mg and 4 mg tablets contain D&C Yellow #10 Aluminum Lake.This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
Mfg. Rev. 01/18
AV Rev. 10/18 (P) - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUANFACINE
guanfacine tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42291-324(NDC:0228-2850) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUANFACINE HYDROCHLORIDE (UNII: PML56A160O) (GUANFACINE - UNII:30OMY4G3MK) GUANFACINE 1 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE (TYPE M) (UNII: U3JF91U133) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) FUMARIC ACID (UNII: 88XHZ13131) GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color orange Score no score Shape ROUND Size 9mm Flavor Imprint Code 850 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42291-324-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200881 07/09/2015 GUANFACINE
guanfacine tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42291-325(NDC:0228-2851) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUANFACINE HYDROCHLORIDE (UNII: PML56A160O) (GUANFACINE - UNII:30OMY4G3MK) GUANFACINE 2 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) FUMARIC ACID (UNII: 88XHZ13131) GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYPROPYL CELLULOSE (TYPE M) (UNII: U3JF91U133) Product Characteristics Color orange Score no score Shape OVAL Size 12mm Flavor Imprint Code 851 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42291-325-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200881 07/09/2015 GUANFACINE
guanfacine tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42291-326(NDC:0228-2853) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUANFACINE HYDROCHLORIDE (UNII: PML56A160O) (GUANFACINE - UNII:30OMY4G3MK) GUANFACINE 3 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) FUMARIC ACID (UNII: 88XHZ13131) GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) HYDROXYPROPYL CELLULOSE (TYPE M) (UNII: U3JF91U133) Product Characteristics Color yellow Score no score Shape ROUND Size 9mm Flavor Imprint Code 853 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42291-326-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200881 07/09/2015 GUANFACINE
guanfacine tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42291-327(NDC:0228-2855) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUANFACINE HYDROCHLORIDE (UNII: PML56A160O) (GUANFACINE - UNII:30OMY4G3MK) GUANFACINE 4 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE (TYPE M) (UNII: U3JF91U133) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) FUMARIC ACID (UNII: 88XHZ13131) GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color yellow Score no score Shape OVAL Size 12mm Flavor Imprint Code 855 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42291-327-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200881 07/09/2015 Labeler - AvKARE (796560394)








