CELLZYME SECRET THERAPY 3845- glycerine, eucalyptusoil, allantoin cream
PHARMACAL-INTERNATIONAL. CO., LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
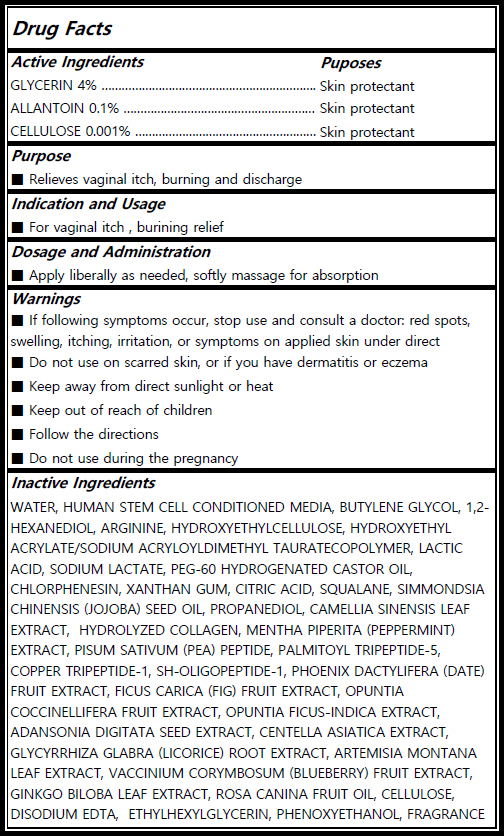
Actvie Ingrdients
GLYCERIN (Skin protectant /4%) ALLANTOIN (Skin protectant/0.1%) CELLULOSE (Skin protectant /0.001%)
Inactives
WATER, HUMAN STEM CELL CONDITIONED MEDIA, BUTYLENE GLYCOL, 1,2-HEXANEDIOL, ARGININE, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATECOPOLYMER, LACTIC ACID, SODIUM LACTATE, PEG-60 HYDROGENATED CASTOR OIL, CHLORPHENESIN, XANTHAN GUM, CITRIC ACID, SQUALANE, SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, PROPANEDIOL, CAMELLIA SINENSIS LEAF EXTRACT, HYDROLYZED COLLAGEN, MENTHA PIPERITA (PEPPERMINT) EXTRACT, PISUM SATIVUM (PEA) PEPTIDE, PALMITOYL TRIPEPTIDE-5, COPPER TRIPEPTIDE-1, SH-OLIGOPEPTIDE-1, PHOENIX DACTYLIFERA (DATE) FRUIT EXTRACT, FICUS CARICA (FIG) FRUIT EXTRACT, OPUNTIA COCCINELLIFERA FRUIT EXTRACT, OPUNTIA FICUS-INDICA EXTRACT, ADANSONIA DIGITATA SEED EXTRACT, CENTELLA ASIATICA EXTRACT, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, ARTEMISIA MONTANA LEAF EXTRACT, VACCINIUM CORYMBOSUM (BLUEBERRY) FRUIT EXTRACT, GINKGO BILOBA LEAF EXTRACT, ROSA CANINA FRUIT OIL, CELLULOSE, DISODIUM EDTA, ETHYLHEXYLGLYCERIN, PHENOXYETHANOL, FRAGRANCE
Indication and Usage
Relieves vaginal itch, burning and discharge.
Dosage and Administration
Apply liberally as needed, softly massage for absorption..
Warnings
If following symptoms occur, stop use and consult a doctor: red spots, swelling, itching, irritation, or symptoms on applied skin under direct sunlight. Keep away from direct sunlight or heat. Keep out of reach of children. Follow the directions. Do not use during the pregnancy.
Keep out of reach of children
Product Labels

PHARMACAL-INTERNATIONAL. CO., LTD

