POVIDONE-IODINE CLEANING SCRUB SWABSTICK 1 AND 3 SWABSTICKS- providone iodine stick
Professional Disposables International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
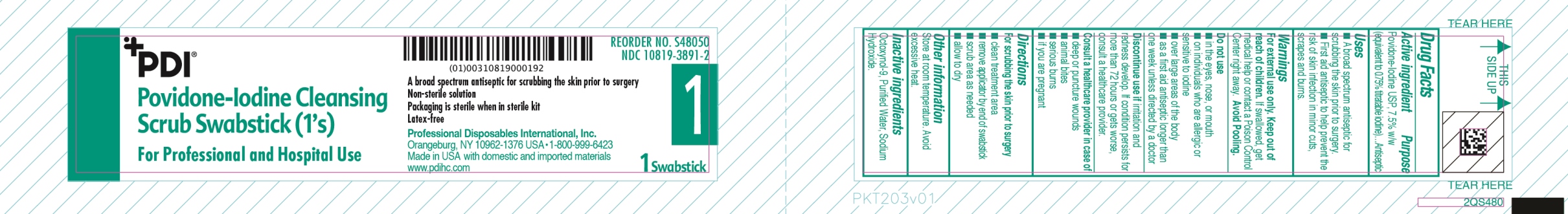
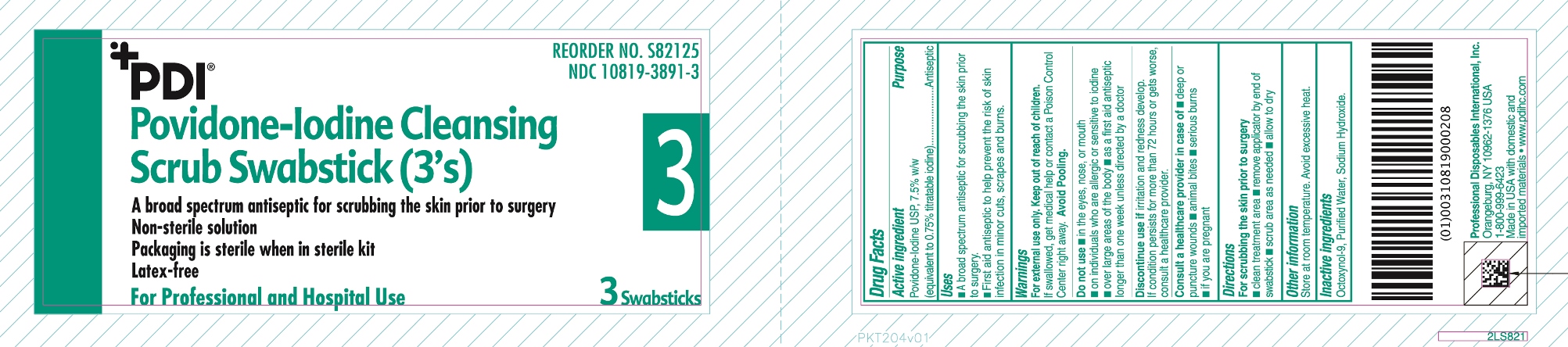
PVP-I Scrub
Uses
- A broad spectrum antiseptic for scrubbing the skin prior to surgery.
- First Aid antiseptic to help prevent the risk of skin infection in minor cut, scrapes and burns.
Warnings
For external use only
Do not use
- in the eyes, nose or mouth
- on individuals who are allergic or sensitive to iodine
- over large areas of the body
- as a first aid antiseptic longer than one week unless directed by a doctor
Discontinue use if irritation and redness develop. If condition persist for more than 72 hours or gets worse, consult a healthcare provider
Consult a healthcare provier in case of
- Deep or puncture wounds
- animal bites
- serious burns
- if you are pregnant
Avoid pooling
Directions
For scrubbing the skin prior to surgery
- clean treatment area
- remove applicator
- scrub area as needed
- allow to dry
| POVIDONE-IODINE CLEANING SCRUB SWABSTICK
1 AND 3 SWABSTICKS
providone iodine stick |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Professional Disposables International, Inc. (800777117) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Professional Disposables International, Inc. | 800777117 | manufacture(10819-3891) | |
Revised: 3/2023
Document Id: f6cd206d-c5df-7924-e053-6294a90a7530
Set id: 300c1c06-4dcd-41f9-bcb9-dec1308e08d6
Version: 6
Effective Time: 20230313
Professional Disposables International, Inc.