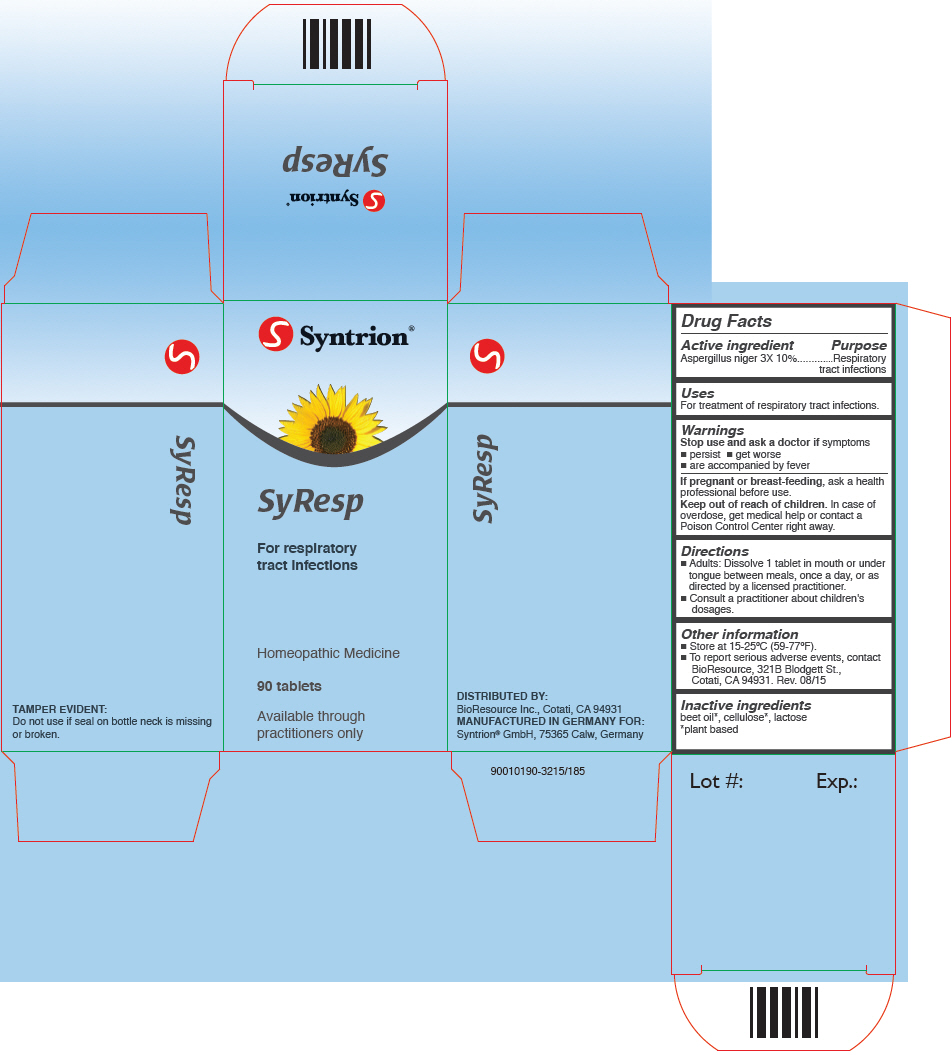
SYRESP- aspergillus niger var. niger tablet
Syntrion GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
SyResp
Directions
- Adults: Dissolve 1 tablet in mouth or under tongue between meals, once a day, or as directed by a licensed practitioner.
- Consult a practitioner about children's dosages.
| SYRESP
aspergillus niger var. niger tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Syntrion GmbH (331883145) |
Revised: 1/2016
Document Id: 5f644c1a-3765-4126-9d41-2bd2c7b7f4b2
Set id: 2f12afb7-c31e-4fc1-a1ef-19aa33d0dcd8
Version: 2
Effective Time: 20160127
Syntrion GmbH