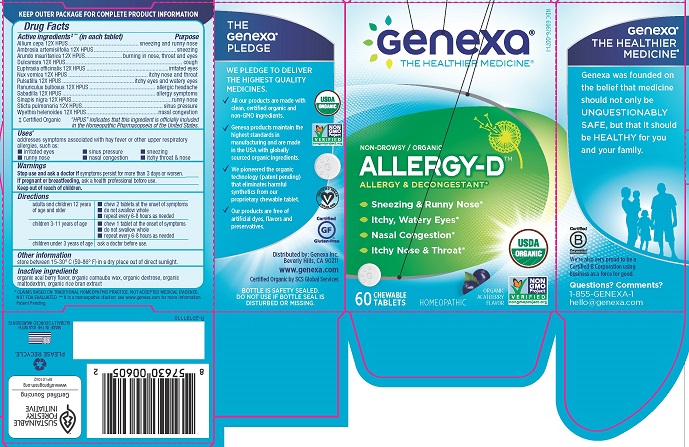
ALLERGY D- allium cepa, ambrosia artemisiifolia, arundo mauritanica, dulcamara, euphrasia officinalis, nux vomica, pulsatilla, ranunculus bulbosus, sabadilla, sinapis nigra, sticta pulmonaria, wyethia helenoides tablet, chewable
Genexa Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ALLERGY-D
Active ingredients#** (in each tablet)
Allium cepa 12X HPUS
Ambrosia artemisiifolia 12X HPUS
Arundo mauritanica 12X HPUS
Dulcamara 12X HPUS
Euphrasia officinalis 12X HPUS
Nux vomica 12X HPUS
Pulsatilla 12X HPUS
Ranunculus bulbosus 12X HPUS
Sabadilla 12X HPUS
Sinapis nigra 12X HPUS
Sticta pulmonaria 12X HPUS
Wyethia helenoides 12X HPUS
# Certified Organic
"HPUS" indicates that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States.
Purpose
sneezing and runny nose
sneezing
burning in nose, throat and eyes
cough
irritated eyes
itchy nose and throat
itchy eyes and watery eyes
allergic headache
allergy symptoms
runny nose
sinus pressure
nasal congestion
Uses*
addresses symptoms associated with hay fever or other upper respiratory allergies, such as:
- irritated eyes
- sinus pressure
- sneezing
- runny nose
- nasal congestion
- itchy throat & nose
Directions
|
adults and children 12 years of age and older |
|
| children 3-11 years of age |
|
| children under 3 years of age | ask a doctor before use |
Inactive ingredients
organic acai berry flavor, organic carnauba wax, organic dextrose, organic maltodextrin, organic rice bran extract
* CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
** X is a homeopathic dilution: see genexa.com for more information
Patent Pending
THE Genexa PLEDGE
WE PLEDGE TO DELIVER THE HIGHEST QUALITY MEDICINES.
All our products are made with clean, certified organic and non-GMO ingredients.
Genexa products maintain the highest standards in manufacturing and are made in the USA with globally sourced organic ingredients.
We pioneered the organic technology (patent pending) that eliminates harmful synthetics from our proprietary chewable tablet.
Our products are free of artificial dyes, flavors and preservatives.
Distributed by:
Genexa Inc.
Beverly Hills, 90211
www. genexa.com
Certified Organic by SCS Global Services
Genexa
THE HEALTHIER MEDICINE
Genexa was founded on the belief that medicine should not only be UNQUESTIONABLY SAFE, but that it should be HEALTHY for you and your family.
We're also very proud to be a Certified B Corporation using business as a force for good.
Questions? Comments?
1-855-GENEXA-1
hello@genexa.com
| ALLERGY D
allium cepa, ambrosia artemisiifolia, arundo mauritanica, dulcamara, euphrasia officinalis, nux vomica, pulsatilla, ranunculus bulbosus, sabadilla, sinapis nigra, sticta pulmonaria, wyethia helenoides tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Genexa Inc. (079751024) |