AC CARE BEES SHIELD BB BROAD SPECTRUM SPF 35- octinoxate, titanium dioxide, zinc oxide cream
DongSung Pharm Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
42361-151 Drug Facts
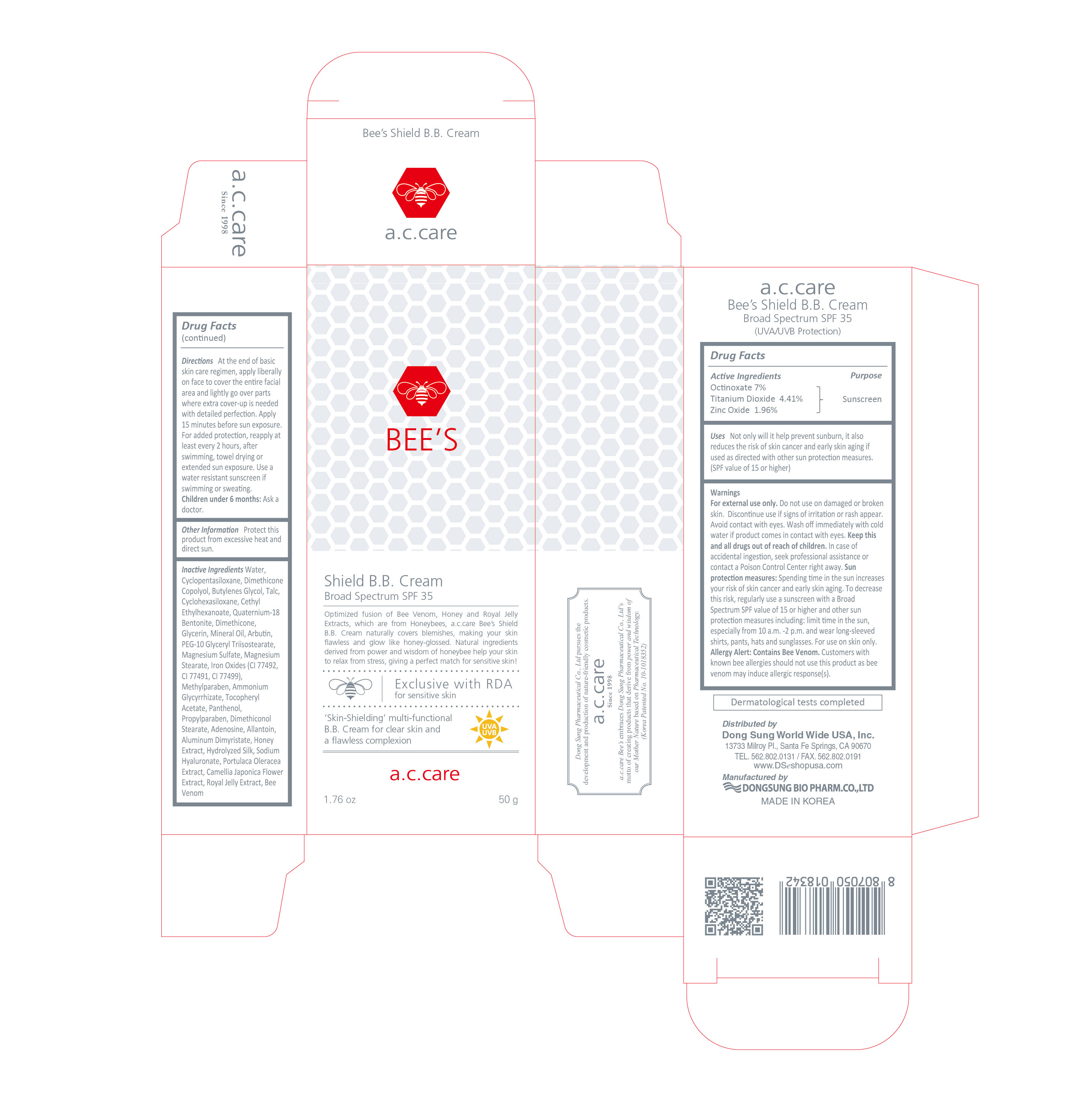
a.c.care BEE'S Shield B.B. Cream
Broad Spectrum SPF 35
Optimized fusion of Bee Venom, Honey and Royal Jelly Extracts, which are from Honeybees, a.c.care Bee's Shield B.B. Cream naturally covers blemishes, making your skin flawless and glow like honey-glossed. Natural ingredients derived from power and wisdom of honeybee help your skin to relax from stress, giving a perfect match for sensitive skin!
'Skin-Shielding' multi-functional B.B. Cream for clear skin and a flawless complexion
Uses
Not only will it help prevent sunburn, it also reduces the risk of skin cancer and early skin aging if used as directed with other sun protection measures. (SPF value of 15 or higher)
Warnings
For external use only. Do not use on damaged or broken skin. Discontinue use if signs of irritation or rash appear. Avoid contact with eyes. Wash off immediately with cold water if product comes in contact with eyes. Keep this and all drugs out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center right away. Sun protection measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. -2 p.m. and wear long-sleeved shirts, pants, hats and sunglasses. For use on skin only. Allergy Alert: Contains Bee Venom. Customers with known bee allergies should not this product as bee venom may induce allergic response(s).
Directions
At the end of basic skin care regimen, apply liberally on face to cover the entire facial area and lightly go over parts where extra cover-up is needed with detailed perfection. Apply 15 minutes before sun exposure. For added protection, reapply at least every 2 hours, after swimming, towel drying or extended sun exposure. Use a water resistant sunscreen if swimming or sweating.
Children under 6 months: Ask a doctor.
Inactive Ingredients
Water, Cyclopentasiloxane, Dimethicone Copolyol, Butylenes Glycol, Talc, Cyclohexasiloxane, Cethyl Ethylhexanoate, Quaternium-18 Bentonite, Dimethicone, Glycerin, Mineral Oil, Arbutin, PEG-10 Glyceryl Triisostearate, Magnesium Sulfate, Magnesium Stearate, Iron Oxides (CI 77492, CI 77491, CI 77499), Methylparaben, Ammonium Glycyrrhizate, Tocopheryl Acetate, Panthenol, Propylparaben, Dimethiconol Stearate, Adenosine, Allantoin, Aluminum Dimyristate, Honey Extract, Hydrolyzed Silk, Sodium Hyaluronate, Portulaca Oleracea Extract, Camellia Japonica Flower Extract, Royal Jelly Extract, Bee Venom
| AC CARE BEES SHIELD BB
BROAD SPECTRUM SPF 35
octinoxate, titanium dioxide, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - DongSung Pharm Co., Ltd. (687856557) |
| Registrant - Dong Sung World Wide USA, Inc. (784969219) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DongSung Pharm Co., Ltd. | 687856557 | label(42361-151) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Omar Sharif Cosmetic Co., Ltd. | 689316318 | manufacture(42361-151) | |