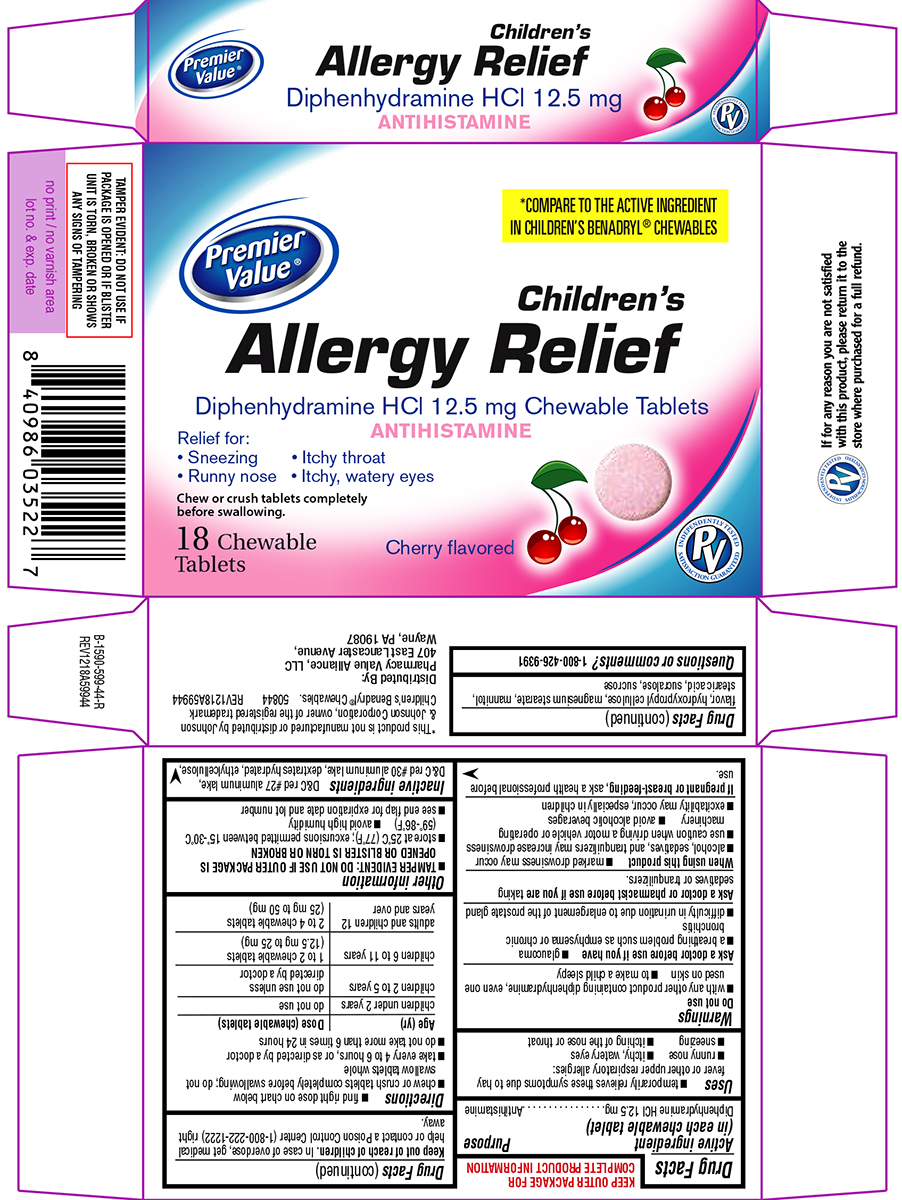
Label: ALLERGY RELIEF CHILDRENS- diphenhydramine hcl tablet, chewable
- NDC Code(s): 68016-051-18
- Packager: Chain Drug Consortium
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
-
Directions
- find right dose on chart below
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
Age (yr) Dose (chewable tablets) children under 2 years do not use children 2 to 5 years do not use unless
directed by a doctorchildren 6 to 11 years 1 to 2 chewable tablets (12.5 mg to 25 mg) adults and children 12 years and over 2 to 4 chewable tablets
(25 mg to 50 mg)
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
Premier
Value®*COMPARE TO THE ACTIVE INGREDIENT
IN CHILDREN’S BENADRYL® CHEWABLESChildren's
Allergy ReliefDiphenhydramine HCl 12.5 mg Chewable Tablets
ANTIHISTAMINERelief for:
• Sneezing • Itchy throat
• Runny nose • Itchy, watery eyesChew or crush tablets completely
before swallowing.18 Chewable
TabletsCherry flavored
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING*This product is not manufactured or distributed by Johnson
& Johnson Corporation, owner of the registered trademark
Children’s Benadryl® Chewables.
50844 REV1218A59944Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087If for any reason you are not satisfied
with this product, please return it to the
store where purchased for a full refund.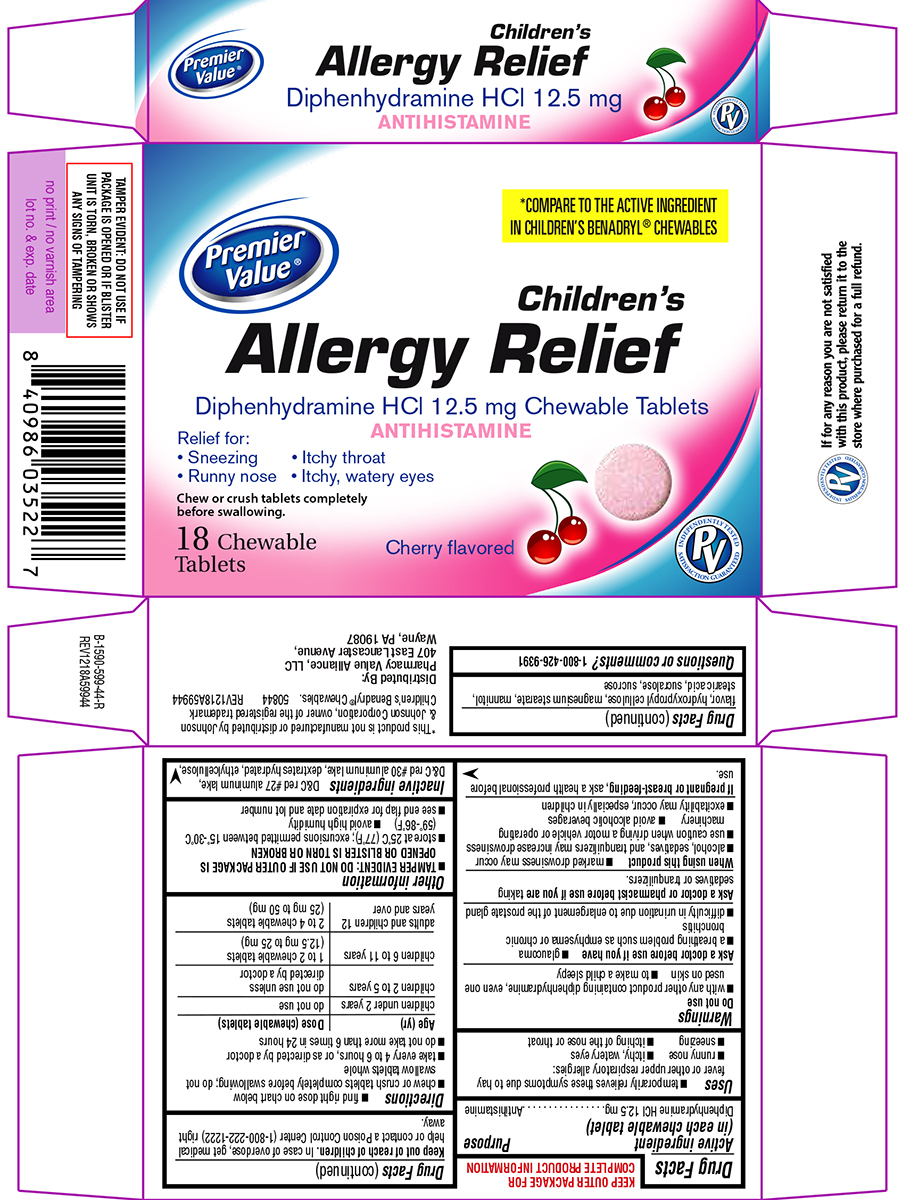
Premier Value 44-599
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF CHILDRENS
diphenhydramine hcl tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-051 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) Product Characteristics Color pink Score no score Shape ROUND Size 12mm Flavor CHERRY Imprint Code 44;599 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-051-18 3 in 1 CARTON 04/25/2011 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/25/2011 Labeler - Chain Drug Consortium (101668460) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(68016-051) , pack(68016-051) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(68016-051)