SINUS CONGESTION- pseudoephedrine hcl tablet, film coated
L. Perrigo Company
----------
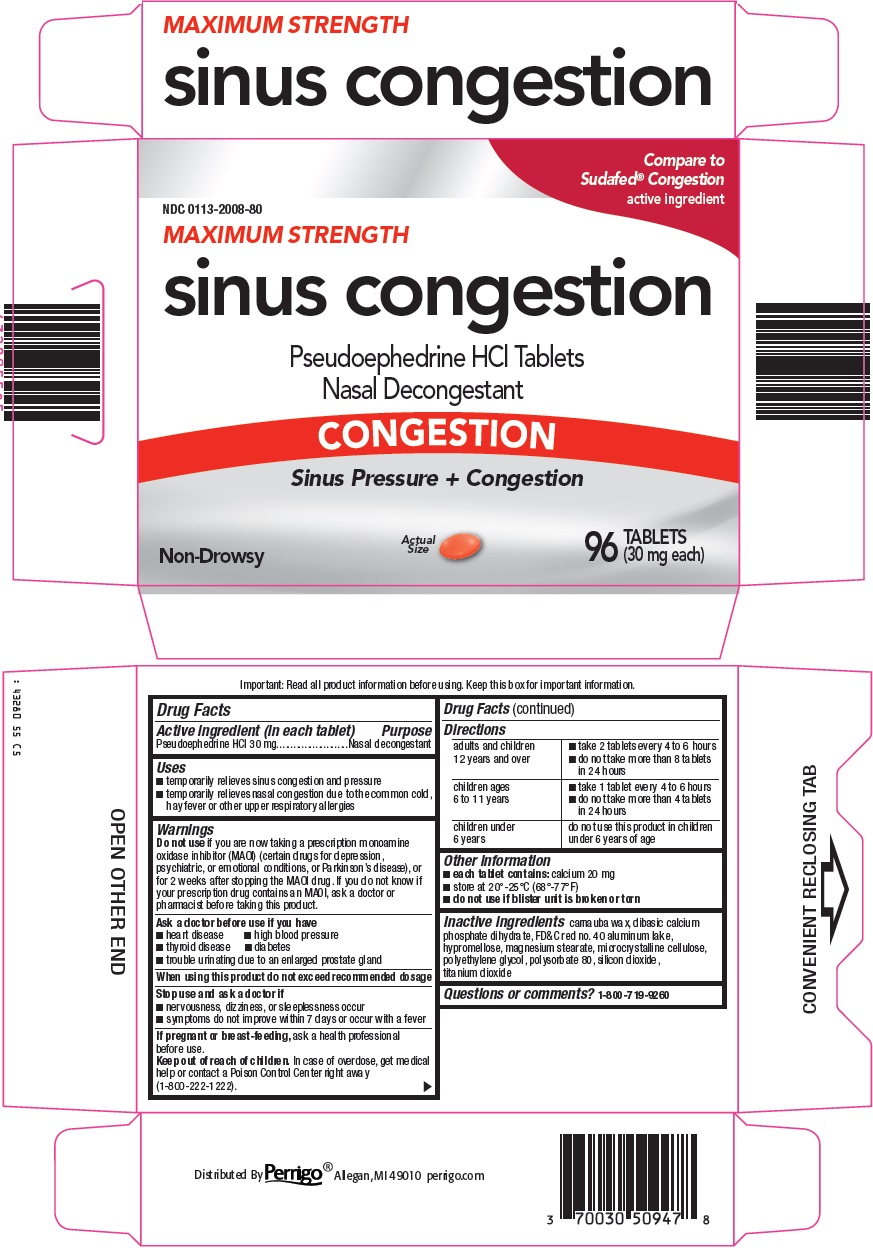
Perrigo Sinus Congestion Drug Facts
Uses
- •
- temporarily relieves sinus congestion and pressure
- •
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
Directions
|
adults and children 12 years and over |
|
|
children ages 6 to 11 years |
|
|
children under 6 years |
do not use this product in children under 6 years of age |
Other information
- •
- each tablet contains: calcium 20 mg
- •
- store at 20°-25°C (68°-77°F)
- •
- do not use if blister unit is broken or torn
| SINUS CONGESTION
pseudoephedrine hcl tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - L. Perrigo Company (006013346) |
Revised: 2/2024
Document Id: b6908caf-e974-4032-a6c5-26c01b0f7e29
Set id: 2dd0e007-4aeb-4b51-815e-4ebe378a4095
Version: 4
Effective Time: 20240221
L. Perrigo Company