SEPIA- sepia tablet
Schwabe North America, Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
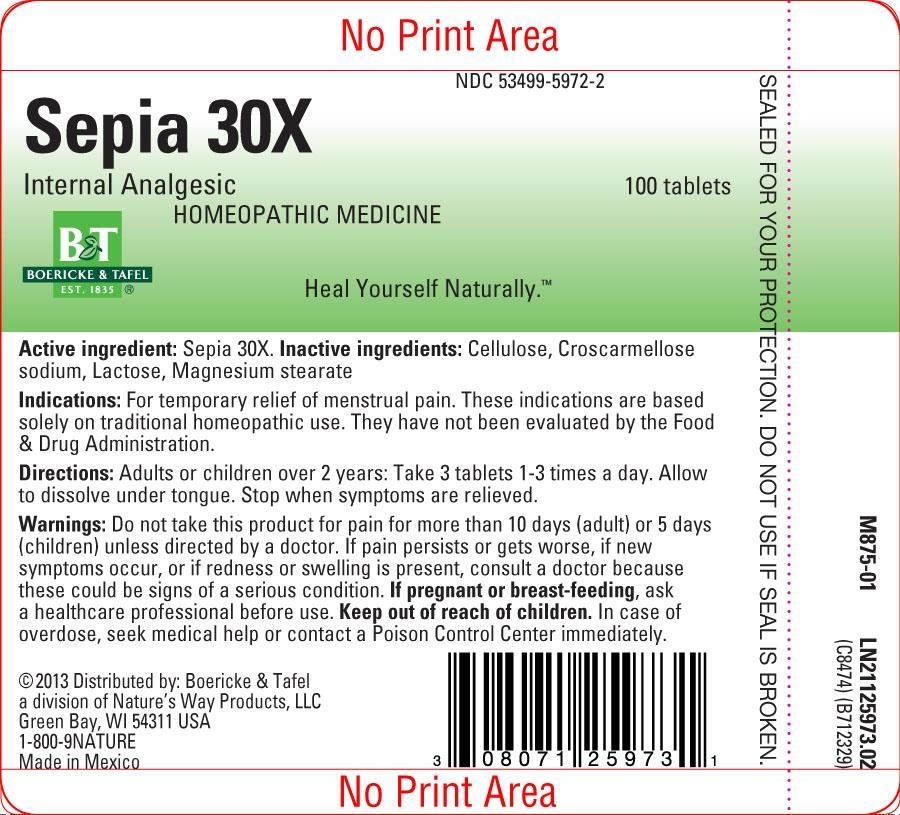
Sepia 30X
Warnings:
Do not take this product for pain for more than 10 days (adult) or 5 days (children) unless directed by a doctor.
If pain persists or gets worse, if new symptoms occur, or if redness or swelling is present, consult a doctor because these could be signs of a serious condition.
Pregnancy or Breast Feeding:
If pregnant or breast-feeding, ask a healthcare professional before use.
| SEPIA
sepia tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Schwabe North America, Inc (831153908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Schwabe Mexico, S.A. de C.V. | 812805901 | manufacture(53499-5972) | |
Revised: 11/2017
Document Id: ec4c0f49-2a99-45e9-91f2-67a0f41cf884
Set id: 2d63d675-cd15-449e-8567-c5a071d9095f
Version: 4
Effective Time: 20171109
Schwabe North America, Inc