UKONIQ- umbralisib tablet, film coated
TG Therapeutics, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use UKONIQ safely and effectively. See full prescribing information for UKONIQ.
UKONIQ™ (umbralisib) tablets, for oral use Initial U.S. Approval: 2021 INDICATIONS AND USAGEUKONIQ is a kinase inhibitor indicated for the treatment of adult patients with:
These indications are approved under accelerated approval based on overall response rate. Continued approval for these indications may be contingent upon verification and description of clinical benefit in a confirmatory trial. DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSTablets: 200 mg ( 3). CONTRAINDICATIONSNone ( 4). WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common (≥15%) adverse reactions, including laboratory abnormalities, were increased creatinine, diarrhea-colitis, fatigue, nausea, neutropenia, transaminase elevation, musculoskeletal pain, anemia, thrombocytopenia, upper respiratory tract infection, vomiting, abdominal pain, decreased appetite, and rash ( 6.1). To report SUSPECTED ADVERSE REACTIONS, contact TG Therapeutics at 1-877-848-9462 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 2/2021 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Marginal Zone Lymphoma
UKONIQ is indicated for the treatment of adult patients with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based regimen.
This indication is approved under accelerated approval based on overall response rate [see Clinical Studies ( 14.1)] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
1.2 Follicular Lymphoma
UKONIQ is indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy.
This indication is approved under accelerated approval based on overall response rate [see Clinical Studies ( 14.2)] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of UKONIQ is 800 mg taken orally once daily with food [see Clinical Pharmacology ( 12.3)] until disease progression or unacceptable toxicity.
Advise patients of the following:
- Swallow tablets whole. Do not crush, break, cut, or chew tablets.
- Take UKONIQ at the same time each day.
- If vomiting occurs, do not take an additional dose; continue with the next scheduled dose.
- If a dose is missed, take a missed dose unless it is less than 12 hours until the next scheduled dose.
2.2 Recommended Prophylaxis
Provide prophylaxis for Pneumocystis jirovecii pneumonia (PJP) during treatment with UKONIQ [see Warnings and Precautions ( 5.1)].
Consider prophylactic antivirals during treatment with UKONIQ to prevent cytomegalovirus (CMV) infection, including CMV reactivation [see Warnings and Precautions ( 5.1)].
2.3 Dosage Modifications for Adverse Reactions
Recommended dosage modifications of UKONIQ for adverse reactions are presented in Table 1 and the recommended dose reductions of UKONIQ for adverse reactions are presented in Table 2.
|
ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; CMV, cytomegalovirus; PJP, Pneumocystis jirovecii pneumonia; ULN, upper limit of normal; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms. aNational Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. |
||
| Adverse Reactions | Severitya | Dosage Modification |
| Hematologic Adverse Reactions | ||
| Neutropenia
[see Warnings and Precautions ( 5.2)] | ANC 0.5 to 1 ×10 9/L |
|
| ANC less than 0.5 × 10 9/L |
|
|
| Thrombocytopenia
[see Adverse Reactions ( 6.1)] | Platelet count 25 to less than 50 × 10
9/L with bleeding
OR Platelet count less than 25 × 10 9/L | Withhold UKONIQ until platelet count 25 × 10
9/L or greater and resolution of bleeding (if applicable), then resume at same dose.
If recurrence, withhold until resolution and then resume at reduced dose. |
| Nonhematologic Adverse Reactions | ||
| Infection, including opportunistic infection
[see Warnings and Precautions ( 5.1)] | Grade 3 or 4 | Withhold UKONIQ until resolved, then resume at same or reduced dose. |
| PJP |
|
|
| CMV infection or viremia | Withhold UKONIQ until infection or viremia resolves, then resume at same or reduced dose. | |
| ALT or AST Elevation
[see Warnings and Precautions ( 5.4)] | AST or ALT greater than 5 to less than 20 times ULN | Withhold UKONIQ until return to less than 3 times ULN, then resume at reduced dose. |
| AST or ALT greater than 20 times
ULN | Discontinue UKONIQ. | |
| Diarrhea or Noninfectious Colitis
[see Warnings and Precautions ( 5.3)] | Mild or moderate diarrhea (up to 6 stools per day over baseline)
OR Asymptomatic (Grade 1) colitis |
|
| Adverse Reactions | Severitya | Dosage Modification |
| Severe diarrhea (greater than 6 stools per day over baseline)
OR Abdominal pain, stool with mucus or blood, change in bowel habits, peritoneal signs |
|
|
| Life-threatening | Discontinue UKONIQ. | |
| Severe Cutaneous
Reactions [see Warnings and Precautions ( 5.5)] | Severe |
|
| Life-threatening | Discontinue UKONIQ. | |
| SJS, TEN, DRESS (any grade) | Discontinue UKONIQ. | |
| Other Adverse
Reactions [see Adverse Reactions ( 6.1)] | Severe | Withhold UKONIQ until resolved, then resume at the same or reduced dose. |
| Life-threatening | Discontinue UKONIQ. | |
| Dose Reduction | Dosage |
| First | 600 mg orally daily |
| Second | 400 mg orally daily |
| Subsequent | Permanently discontinue UKONIQ in patients unable to tolerate 400 mg orally daily |
3 DOSAGE FORMS AND STRENGTH
Tablets: 200 mg, green film-coated, oval-shaped with “L474” on one side and plain on the other side.
5 WARNINGS AND PRECAUTIONS
5.1 Infections
Serious, including fatal, infections occurred in patients treated with UKONIQ. Grade 3 or higher infections occurred in 10% of 335 patients, with fatal infections occurring in <1%. The most frequent Grade ≥3 infections included pneumonia, sepsis, and urinary tract infection. The median time to onset of Grade ≥3 infection was 2.4 months (range: 1 day to 21 months) [see Adverse Reactions ( 6.1)].
Monitor for any new or worsening signs and symptoms of infection. For Grade 3 or 4 infection, withhold UKONIQ until infection has resolved. Resume UKONIQ at the same or a reduced dose [see Dosage and Administration ( 2.3)].
Provide prophylaxis for Pneumocystis jirovecii pneumonia (PJP) during treatment with UKONIQ [see Dosage and Administration ( 2.2)] . Withhold UKONIQ in patients with suspected PJP of any grade and permanently discontinue in patients with confirmed PJP [see Dosage and Administration ( 2.2)] .
Monitor for cytomegalovirus (CMV) infection during treatment with UKONIQ in patients with a history of CMV infection. Consider prophylactic antivirals during treatment with UKONIQ to prevent CMV infection, including CMV reactivation [see Dosage and Administration ( 2.2)]. For clinical CMV infection or viremia, withhold UKONIQ until infection or viremia resolves. If UKONIQ is resumed, administer the same or reduced dose and monitor patients for CMV reactivation by PCR or antigen test at least monthly [see Dosage and Administration ( 2.2)] .
5.2 Neutropenia
Serious neutropenia occurred in patients treated with UKONIQ. Grade 3 neutropenia developed in 9% of 335 patients and Grade 4 neutropenia developed in 9% [see Adverse Reactions ( 6.1)]. The median time to onset of Grade 3 or 4 neutropenia was 45 days.
Monitor neutrophil counts at least every 2 weeks for the first 2 months of UKONIQ and at least weekly in patients with neutrophil counts <1 ×10 9/L (Grade 3-4). Consider supportive care as appropriate. Withhold, reduce dose, or discontinue UKONIQ depending on the severity and persistence of neutropenia [see Dosage and Administration ( 2.3)].
5.3 Diarrhea or Non-infectious Colitis
Serious diarrhea or non-infectious colitis occurred in patients treated with UKONIQ. Any grade diarrhea or colitis occurred in 53% of 335 patients and Grade 3 occurred in 9% [see Adverse Reactions ( 6.1)]. The median time to onset for any grade diarrhea or colitis was 1 month (range: 1 day to 23 months), with 75% of cases occurring by 2.9 months.
For patients with severe diarrhea (Grade 3, i.e., > 6 stools per day over baseline) or abdominal pain, stool with mucus or blood, change in bowel habits, or peritoneal signs, withhold UKONIQ until resolved and provide supportive care with antidiarrheals or enteric acting steroids as appropriate. Upon resolution, resume UKONIQ at a reduced dose. For recurrent Grade 3 diarrhea or recurrent colitis of any grade, discontinue UKONIQ. Discontinue UKONIQ for lifethreatening diarrhea or colitis [see Dosage and Administration ( 2.3)].
5.4 Hepatotoxicity
Serious hepatotoxicity occurred in patients treated with UKONIQ. Grade 3 and 4 transaminase elevations (ALT and/or AST) occurred in 8% and <1%, respectively, in 335 patients [see Adverse Reactions ( 6.1)]. The median time to onset for Grade 3 or higher transaminase elevations was 2.2 months (range: 15 days to 4.7 months).
Monitor hepatic function at baseline and during treatment with UKONIQ. For ALT/AST greater than 5 to less than 20 times ULN, withhold UKONIQ until return to less than 3 times ULN, then resume at a reduced dose. For ALT/AST elevation greater than 20 times ULN, discontinue UKONIQ [see Dosage and Administration ( 2.3)] .
5.5 Severe Cutaneous Reactions
Severe cutaneous reactions, including a fatal case of exfoliative dermatitis, occurred in patients treated with UKONIQ. Grade 3 cutaneous reactions occurred in 2% of 335 patients and included exfoliative dermatitis, erythema, and rash (primarily maculo-papular) [see Adverse Reactions ( 6.1)] . The median time to onset of Grade 3 or higher cutaneous reaction was 15 days (range: 9 days to 6.4 months).
Monitor patients for new or worsening cutaneous reactions. Review all concomitant medications and discontinue any potentially contributing medications. Withhold UKONIQ for severe (Grade 3) cutaneous reactions until resolution. Monitor at least weekly until resolved. Upon resolution, resume UKONIQ at a reduced dose. Discontinue UKONIQ if severe cutaneous reaction does not improve, worsens, or recurs. Discontinue UKONIQ for life-threatening cutaneous reactions or SJS, TEN, or DRESS of any grade [see Dosage and Administration ( 2.3)]. Provide supportive care as appropriate.
5.6 Allergic Reactions Due to Inactive Ingredient FD&C Yellow No. 5
UKONIQ contains FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions
(including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
5.7 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, UKONIQ can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of umbralisib to pregnant mice during the period of organogenesis caused adverse developmental outcomes including embryo-fetal mortality and fetal malformations at maternal exposures comparable to those in patients at the recommended dose of 800 mg. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment and for one month after the last dose [see Use in Specific Populations ( 8.1, 8.3)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infections [see Warnings and Precautions ( 5.1)]
- Neutropenia [see Warnings and Precautions ( 5.2)]
- Diarrhea and Non-infectious Colitis [see Warnings and Precautions ( 5.3)]
- Hepatotoxicity [see Warnings and Precautions ( 5.4)]
- Severe Cutaneous Reactions [see Warnings and Precautions ( 5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be compared to rates in the clinical trials of another drug and may not reflect the rates observed in the general patient population.
The pooled safety population described in WARNINGS AND PRECAUTIONS reflects exposure to UKONIQ as monotherapy at a dosage of 800 mg orally once daily in 335 adults with hematologic malignancies in studies TGR-1202-101, TGR-1202-202, UTX-TGR-205, and UTX-TGR-501. Among these 335 patients who received UKONIQ, 52% were exposed for 6 months or longer and 30% were exposed for greater than one year.
Relapsed or Refractory Follicular Lymphoma and Marginal Zone Lymphoma
The safety of UKONIQ was evaluated in a pooled safety population that included 221 adults with marginal zone lymphoma (37%) and follicular lymphoma (63%) enrolled in three single-arm, open-label trials (Study TGR-1202-101, TGR-1202-202, and UTX-TGR-205) and one open-label extension trial (Study UTX-TGR-501) [see Clinical Studies ( 14.1, 14.2)] . These trials required hepatic transaminases ≤ 2.5 times upper limit of normal (ULN), total bilirubin ≤ 1.5 times ULN, and creatinine clearance ≥ 30 mL/min. No patients had prior exposure to a PI3K inhibitor. Patients received UKONIQ 800 mg orally once daily. Among these 221 patients who received UKONIQ, 60% were exposed for 6 months or longer and 34% were exposed for greater than one year.
The median age was 66 years (range: 29 to 88 years), 43% were female, and 97% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. Race was reported in 92% of patients; of these patients, 89% were White, 6% were Black, and 3% were Asian. Patients had a median of 2 prior therapies (range 1 to 10).
Serious adverse reactions occurred in 18% of patients who received UKONIQ. Serious adverse reactions that occurred in ≥2% of patients were diarrhea-colitis (4%), pneumonia (3%), sepsis (2%), and urinary tract infection (2%). Fatal adverse reactions occurred in <1% of patients who received UKONIQ, including exfoliative dermatitis.
Permanent discontinuation of UKONIQ due to an adverse reaction occurred in 14% of patients. Adverse reactions which resulted in permanent discontinuation of UKONIQ in ≥5% of patients included diarrhea-colitis (6%) and transaminase elevation (5%).
Dose reductions of UKONIQ due to an adverse reaction occurred in 11% of patients. Adverse reactions which required dose reductions in ≥4% of patients included diarrhea-colitis (4%).
Dosage interruptions of UKONIQ due to an adverse reaction occurred in 43% of patients. Adverse reactions which required dosage interruption in ≥5% of patients included diarrhea-colitis (18%), transaminase elevation (7%), neutropenia (5%), vomiting (5%), and upper respiratory tract infection (5%).
The most common (≥15%) adverse reactions, including laboratory abnormalities, were increased creatinine, diarrhea-colitis, fatigue, nausea, neutropenia, transaminase elevation, musculoskeletal pain, anemia, thrombocytopenia, upper respiratory tract infection, vomiting, abdominal pain, decreased appetite, and rash.
Table 3 provides the adverse reactions in the pooled safety population of 221 patients with marginal zone lymphoma and follicular lymphoma who received the recommended dosage.
|
aAbdominal pain includes Abdominal pain, abdominal pain upper, abdominal pain lower, abdominal discomfort |
||
|
bFatigue includes Fatigue, asthenia, lethargy |
||
|
cEdema includes Edema peripheral, face edema, pulmonary edema, fluid overload, generalized edema dMusculoskeletal pain includes Back pain, myalgia, pain in extremity, musculoskeletal pain, neck pain, spinal pain, musculoskeletal chest pain, musculoskeletal discomfort |
||
|
eUpper respiratory tract infection includes Upper respiratory tract infection, sinusitis, nasopharyngitis, rhinitis |
||
|
fRash includes Rash, rash maculo-papular, rash erythematous, rash pruritic, rash macular, exfoliative dermatitis |
||
| Adverse Reactions | UKONIQ
N=221 |
|
| All Grades
(%) | Grade 3 or 4
(%) |
|
| Gastrointestinal Disorders | ||
| Diarrhea | 58 | 10 |
| Nausea | 38 | <1 |
| Vomiting | 21 | <1 |
| Abdominal pain a | 19 | 3 |
| General Disorders and Administration Site Conditions | ||
| Fatigue b | 41 | 3 |
| Edema c | 14 | <1 |
| Pyrexia | 10 | 0 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Musculoskeletal pain d | 27 | 2 |
| Infections | ||
| Upper respiratory tract infection e | 21 | <1 |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 19 | 2 |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash f | 18 | 3 |
| Psychiatric Disorders | ||
| Insomnia | 14 | <1 |
Clinically relevant adverse reactions in <10% of patients who received UKONIQ included urinary tract infection (9%), dyspnea (7%), pneumonia (6%), sepsis (3%), colitis (2%), pneumonitis (<1%), and exfoliative dermatitis (<1%).
Table 4 provides the laboratory abnormalities in the pooled safety population of 221 patients with marginal zone lymphoma and follicular lymphoma who received the recommended dosage.
|
aLaboratory values were categorized using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03 grading system. |
|||
| Laboratory Parameter | UKONIQ
N=221 |
||
| All Gradesa
(%) | Grade 3 or 4
(%) |
||
| Hematologic
|
|
|
|
| Neutrophil decreased
| 33
| 16
|
|
| Hemoglobin decreased
| 27
| 3
|
|
| Platelets decreased | 26 | 4 | |
| Chemistry
|
|
|
|
| Creatinine increased
| 79
| 0
|
|
| Alanine aminotransferase increased
| 33
| 8
|
|
| Aspartate aminotransferase increased
| 32
| 7
|
|
| Potassium decreased | 21 | 4 | |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and the mechanism of action [see Clinical Pharmacology ( 12.1)] , UKONIQ can cause fetal harm when administered to a pregnant woman. There are no available data on UKONIQ use in pregnant women to evaluate for a drug-associated risk. In animal reproduction studies, administration of umbralisib to pregnant mice during organogenesis resulted in adverse developmental outcomes, including alterations to growth, embryo-fetal mortality, and structural abnormalities at maternal exposures (AUC) comparable to those in patients at the recommended dose of 800 mg (see Data) . Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study in mice, pregnant animals were administered oral doses of umbralisib at 100, 200, and 400 mg/kg/day during the period of organogenesis. Malformations were observed at doses of 200 mg/kg/day (cleft palate) and 400 mg/kg/day (cleft palate and nasopharyngeal fistula). Additional findings occurred starting at the dose of 100 mg/kg/day and included folded retina, delayed ossification of sternebrae and vertebrae, increased resorptions, and increased post-implantation loss. The exposure (AUC) at a dose of 100 mg/kg/day in mice is approximately equivalent to the human exposure at the recommended dose of 800 mg.
In an embryo-fetal development study in rabbits, pregnant animals were administered oral doses of umbralisib at 30, 100, and 300 mg/kg/day during the period of organogenesis. Administration at 300 mg/kg/day resulted in maternal toxicity (decreased food consumption and body weight) and reduced fetal weights. The exposure (AUC) at 300 mg/kg/day in rabbits is approximately 0.03 times the exposure in human patients at the recommended dose of 800 mg.
8.2 Lactation
Risk Summary
There are no data on the presence of umbralisib in human milk or the effects on the breastfed child or milk production. Because of the potential for serious adverse reactions from umbralisib in the breastfed child, advise women not to breastfeed during treatment with UKONIQ and for one month after the last dose.
8.3 Females and Males of Reproductive Potential
UKONIQ may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating UKONIQ.
Contraception
Infertility
Males
Based on the findings from mice and dogs, UKONIQ may impair male fertility [see Nonclinical Toxicology ( 13.1)]. Trend for reversibility was noted in dogs 30 days after the last dose.
8.4 Pediatric Use
Safety and effectiveness of UKONIQ have not been established in pediatric patients.
8.5 Geriatric Use
Of the 221 patients with MZL or FL who received UKONIQ in clinical studies, 56% of patients were 65 years of age and older, while 19% were 75 years of age and older. No overall differences in effectiveness or pharmacokinetics were observed between these patients and younger patients. In patients 65 years of age and older, 23% experienced serious adverse reactions compared to 12% in patients younger than 65 years of age. There was a higher incidence of infectious serious adverse reactions in patients 65 years of age or older (13%) compared to patients younger than 65 years of age (4%).
8.6 Renal Impairment
No dose adjustment is recommended in patients with mild or moderate renal impairment
(creatinine clearance [CLcr] 30 to 89 mL/min estimated by Cockcroft-Gault equation) [see Clinical Pharmacology ( 12.3)] . UKONIQ has not been studied in patients with severe renal impairment ([CLcr] < 30 mL/min).
8.7 Hepatic Impairment
No dose adjustment is recommended for patients with mild hepatic impairment (total bilirubin ≤ upper limit of normal [ULN] and AST > ULN or total bilirubin >1 to 1.5 × ULN and any AST) [see Clinical Pharmacology ( 12.3)] . UKONIQ has not been studied in patients with moderate (total bilirubin > 1.5 to 3 × ULN and any AST) or severe hepatic impairment (total bilirubin > 3 × ULN and any AST).
11. DESCRIPTION
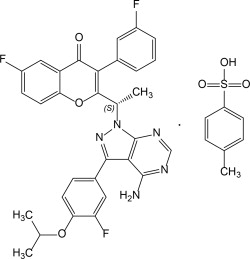
Umbralisib is a kinase inhibitor. The active pharmaceutical ingredient is umbralisib tosylate with the molecular formula C 38H 32F 3N 5O 6S and a molecular weight of 743.75 g/mol. The chemical name for umbralisib tosylate is ( S)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxyphenyl)-1 H-pyrazolo
[3, 4-d] pyrimidin-1-yl)-ethyl)-6-fluoro-3-(3-fluorophenyl)-4 H-chromen-4-one 4methylbenzenesulfonate and has the following structure:
Umbralisib tosylate is white to light brown powder that is freely soluble in dimethyl sulfoxide, soluble in methanol, and practically insoluble in water. The ionization constant (pKa) of umbralisib tosylate is 2.71.
UKONIQ tablets are for oral administration. Each tablet contains 200 mg of umbralisib free base equivalent to 260.2 mg of umbralisib tosylate. The tablets also contain inactive ingredients: croscarmellose sodium, hydroxypropyl betadex, hydroxypropyl cellulose, magnesium stearate and microcrystalline cellulose.
The tablet coating film consists of FD&C Blue No. 1, FD&C Yellow No. 5, ferric oxide yellow, hypromellose 2910, polydextrose, polyethylene glycol 8000, titanium dioxide and triacetin.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Umbralisib inhibits multiple kinases. In biochemical and cell-based assays, umbralisib inhibited PI3Kδ and casein kinase CK1ε. PI3Kδ is expressed in normal and malignant B-cells; CK1ε has been implicated in the pathogenesis of cancer cells, including lymphoid malignancies. Umbralisib also inhibited a mutated form of ABL1 in biochemical assays. Umbralisib inhibited cell proliferation, CXCL12-mediated cell adhesion, and CCL19-mediated cell migration in lymphoma cell lines in studies conducted in vitro.
12.2 Pharmacodynamics
Exposure-Response Relationships
An exposure-response relationship between umbralisib and overall response rate was observed in patients with FL. The time course of pharmacodynamic response is unknown.
A relationship between higher umbralisib steady state exposures and higher incidence of adverse reactions, including diarrhea (any grade) and elevated AST/ALT (any grade and Grade ≥ 3), was observed.
12.3 Pharmacokinetics
Umbralisib exposures increased proportionally over a dose range of 200 mg to 1000 mg once daily (0.25 to 1.25 times the recommended dosage). A 6.4- and 3.8-fold accumulation of AUC and C max of umbralisib, respectively, were observed at the recommended dosage.
The mean (CV%) steady-state AUC and C max were 141 μg*h/mL (46%) and 7.3 μg/mL (39%), respectively, at the recommended dosage.
Absorption
The median time to reach peak plasma concentration (t max) is approximately 4 hours.
Effect of Food
Administration of a single dose of UKONIQ with a high-fat, high calorie meal (approximately 917 calories with 171 calories from protein, 232 calories from carbohydrate, and 502 calories from fat) in healthy subjects increased AUC and C max of umbralisib by 61% and 115%, respectively, relative to fasting conditions.
Distribution
The mean (CV%) apparent central volume of distribution of umbralisib is 312 (185%) L.
The plasma protein binding is ≥99.7% and was independent of concentration between 2 and 5 μM. Mean blood-to-plasma ratio is 0.6.
Elimination
The mean (CV%) apparent clearance of umbralisib is 15.5 (52%) L/h with effective half-life of 91 (42%) hours.
Excretion
Approximately 81% of the dose was recovered in feces (17% unchanged) and 3% in urine (0.02% unchanged) following a single radiolabeled dose of umbralisib 800 mg to healthy subjects.
Specific Populations
No clinically significant differences in the pharmacokinetics of umbralisib were observed based on age (18 to 87 years old), sex, race (White and Black), body weight (44 to 165 kg), mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin >1 to 1.5 × ULN and any AST), or mild and moderate renal impairment (CLcr 30 to 89 mL/min, estimated by the Cockcroft-Gault equation). The effect of other race/ethnicity, severe renal impairment (CLcr 15 to 29 mL/min) and dialysis, or moderate to severe hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin > ULN and any AST) on umbralisib pharmacokinetics is unknown.
Drug Interaction Studies
Clinical Studies
No clinically significant differences in umbralisib pharmacokinetics were observed when used concomitantly with omeprazole, a proton pump inhibitor.
Coadministration of UKONIQ with strong CYP3A4 inhibitors and inducers or moderate CYP2C9 inhibitors and inducers have not been studied.
Coadministration of UKONIQ with sensitive substrates of CYP3A4, CYP2C8, CYP2C9 and CYP2C19, or P-gp substrates have not been fully characterized.
In Vitro Studies
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with umbralisib.
Umbralisib was not mutagenic in a bacterial mutagenicity (Ames) assay. Umbralisib was not clastogenic in an in vitro micronucleus assay using human (TK6) lymphoblast cells or in an in vivo micronucleus assay in mice at doses up to 1000 mg/kg.
In a repeat-dose toxicology study in dogs, adverse findings in reproductive organs were observed in males and included findings in the testis (decreased weight, tubular degeneration, tubular atrophy) and epididymis (decreased weight, luminal debris) at doses ≥100 mg/kg, which is approximately 0.1 times the human exposure (AUC) at the recommended dose of 800 mg. In a combined male and female fertility study in mice, umbralisib was administered orally at doses of 50, 150, or 300 mg/kg/day, starting 28 days prior to pairing and through mating in males and starting 14 days prior to pairing through Gestation Day 7 in females. Adverse findings were observed at doses of 150 and 300 mg/kg/day and included decreased testicular and epididymis weights, decreased sperm mobility and counts, increased post-implantation loss, and increased resorption. The dose of 150 mg/kg results in an exposure approximately 1.3 times the human exposure (AUC) at the recommended dose of 800 mg.
14 CLINICAL STUDIES
14.1 Marginal Zone Lymphoma
The efficacy of UKONIQ was evaluated in a single-arm cohort of Study UTX-TGR-205 (NCT02793583), an open-label, multi-center, multi-cohort trial. Patients with MZL were required to have received at least one prior therapy, including an anti-CD20 containing regimen. The trial excluded patients with prior exposure to a PI3K inhibitor. Patients received UKONIQ 800 mg orally once daily until disease progression or unacceptable toxicity.
A total of 69 patients with MZL [extranodal (N=38), nodal (N=20), and splenic (N=11)] were enrolled in this cohort. The median age was 67 years (range: 34 to 88 years), 52% were female, 83% were White, 7% were Black, 3% were Asian, 7% were Other, and 97% had a baseline ECOG performance status of 0 or 1. Patients had a median number of prior lines of therapy of 2 (range: 1 to 6), with 26% being refractory to their last therapy.
Efficacy was based on overall response rate as assessed by an Independent Review Committee (IRC) using criteria adopted from the International Working Group criteria for malignant lymphoma. The median follow-up time was 20.3 months (range: 15.0 to 28.7 months). Efficacy results are shown in Table 5.
|
CI, confidence interval; CR, complete response; DOR, duration of response; IRC, Independent Review Committee; ORR, overall response rate; NE, not evaluable; NR, not reached; PR, partial response. |
|
|
a Per IRC according to Revised International Working Group Criteria |
|
|
b Based on Kaplan-Meier estimation |
|
|
+ Denotes censored observation |
|
| Endpoint | Total
(N=69) |
| ORR, n (%) a | 34 (49) |
| 95% CI | 37.0, 61.6 |
| CR, n (%) | 11 (16) |
| PR, n (%) | 23 (33) |
| DOR | |
| Median, months (95% CI) b | NR (9.3, NE) |
| Range, months | 0.0 +, 21.8 + |
The median time to response was 2.8 months (range: 1.8 to 21.2 months). Overall response rates were 44.7%, 60.0%, and 45.5% for the 3 MZL sub-types (extranodal, nodal, and splenic, respectively).
14.2 Follicular Lymphoma
The efficacy of UKONIQ was evaluated in a single-arm cohort of Study UTX-TGR-205, an open-label, multi-center, multi-cohort trial (NCT02793583). Patients with relapsed or refractory FL were required to have received at least two prior systemic therapies, including an anti-CD20 monoclonal antibody and an alkylating agent. The trial excluded patients with Grade 3b FL, large cell transformation, prior allogeneic transplant, history of CNS lymphoma, and prior exposure to a PI3K inhibitor. Patients received UKONIQ 800 mg orally once daily until disease progression or unacceptable toxicity.
A total of 117 patients with FL were enrolled in this cohort. The median age was 65 years
(range: 29 to 87 years), 38% were female, 80% were White, 4% were Black, 73% had Stage III-IV disease, 38% had bulky disease and 97% had a baseline ECOG performance status of 0 to 1. Patients had a median of 3 prior lines of therapy (range: 1 to 10), with 36% refractory to their last therapy.
Efficacy was based on overall response rate as assessed by an Independent Review Committee (IRC) using criteria adopted from the International Working Group criteria for malignant lymphoma. The median follow-up time was 20.1 months (range: 13.5 to 29.6 months). Efficacy results are shown in Table 6.
|
CI, confidence interval; CR, complete response; DOR, duration of response; IRC, Independent Review Committee; ORR, overall response rate; PR, partial response. |
|
|
a Per IRC according to Revised International Working Group Criteria |
|
|
b Based on Kaplan-Meier estimation |
|
|
+ Denotes censored observation |
|
| Endpoint | Total (N=117) |
| ORR, n (%) a | 50 (43) |
| 95% CI | 33.6, 52.2 |
| CR, n (%) | 4 (3.4) |
| PR, n (%) | 46 (39) |
| DOR | |
| Median months (95% CI) b | 11.1 (8.3, 16.4) |
| Range, months | 0.0 +, 20.9 + |
The median time to response was 4.4 months (range: 2.2 to 15.5 months).
16 HOW SUPPLIED/STORAGE AND HANDLING
UKONIQ tablets are supplied as follows:
| Tablet Strength | Description | Package Configuration | NDC Number |
| 200 mg | Green, film-coated, oval-shaped tablets debossed with “L474” on one side and plain on the other side | White opaque round 150cc high density polyethylene (HDPE) bottle capped with a 38 mm child resistant polypropylene closure with heat sealed peelable foil liner.
Each bottle contains 120 tablets | 73150-200-12 |
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling ( Medication Guide).
Infections
Advise patients that UKONIQ can cause serious infections that may be fatal. Advise patients to immediately report any signs or symptoms of infection (e.g., fever, chills, weakness) [see Warnings and Precautions ( 5.1)].
Neutropenia
Advise patients of the need for periodic monitoring of blood counts and to notify their healthcare provider immediately if they develop a fever or any signs of infection [see Warnings and Precautions ( 5.2)] .
Diarrhea or Non-Infectious Colitis
Advise patients that they may experience loose stools or diarrhea and should contact their healthcare provider with any persistent or worsening diarrhea. Advise patients to maintain adequate hydration [see Warnings and Precautions ( 5.3)] .
Advise patients of the possibility of colitis and to notify their healthcare provider of any abdominal pain/distress [see Warnings and Precautions ( 5.3)] .
Hepatotoxicity
Advise patients that UKONIQ may cause significant elevations in liver enzymes and the need for periodic monitoring of liver tests. Advise patients to report symptoms of liver dysfunction including jaundice (yellow eyes or yellow skin), abdominal pain, bruising, or bleeding [see Warnings and Precautions ( 5.4)] .
Severe Cutaneous Reactions
Advise patients that UKONIQ may cause a severe skin rash and to notify their healthcare provider immediately if they develop a new or worsening skin rash [see Warnings and Precautions ( 5.5)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions ( 5.7) , Use in Specific Populations ( 8.1, 8.3)].
Advise females of reproductive potential to use effective contraceptive during treatment with UKONIQ and for one month after the last dose [see Use in Specific Populations ( 8.3)] .
Advise males with female partners of reproductive potential to use effective contraceptive during treatment with UKONIQ and for one month after the last dose [see Use in Specific Populations ( 8.3)] .
Lactation
Advise women not to breastfeed during treatment with UKONIQ and for one month after the last dose [see Use in Specific Populations ( 8.2)] .
Infertility
Advise males of reproductive potential that UKONIQ may impair fertility [see Use in Specific Populations ( 8.3)].
Allergic Reactions Due to Inactive Ingredient FD&C Yellow No. 5
Advise patients that UKONIQ contains FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions in certain susceptible persons [see Warnings and Precautions ( 5.6)].
Administration
Inform patients to take UKONIQ orally once daily at approximately the same time each day with food and how to make up a missed or vomited dose. Advise patients to swallow tablets whole. Advise patients not to crush, break, cut or chew tablets [see Dosage and Administration ( 2.1)] .
Distributed by:
TG Therapeutics, Inc.
343 Thornall Street, Suite 740
Edison, NJ 08837
For patent information: https://www.tgtherapeutics.com/our-products/patent/
UKONIQ™ is a trademark of TG Therapeutics, Inc.
© TG Therapeutics, Inc. 2020
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Issued: 02/2021 |
|
| MEDICATION GUIDE
UKONIQ™ (you-KON-ik) (umbralisib) Tablets |
||
|
What is the most important information I should know about UKONIQ?
|
||
| If you have any of the above serious side effects during treatment with UKONIQ, your doctor may completely stop your treatment, stop your treatment for a period of time, or change your dose of UKONIQ.
See “ What are the possible side effects of UKONIQ?” for more information about side effects. |
||
| What is UKONIQ?
UKONIQ is a prescription medicine used to treat adults with:
|
||
| It is not known if UKONIQ is safe and effective in children. | ||
Before taking UKONIQ, tell your healthcare provider about all of your medical conditions, including if you:
|
||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
How should I take UKONIQ?
|
||
|
What are the possible side effects of UKONIQ?
|
||
|
|
|
| These are not all of the possible side effects of UKONIQ.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store UKONIQ?
|
||
| Keep UKONIQ and all medicines out of reach of children. | ||
| General information about the safe and effective use of UKONIQ.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use UKONIQ for a condition for which it was not prescribed. Do not give UKONIQ to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about UKONIQ that is written for healthcare professionals. |
||
| What are the ingredients in UKONIQ?
Active ingredient: umbralisib tosylate Inactive ingredients: croscarmellose sodium, hydroxypropyl betadex, hydroxypropyl cellulose, magnesium stearate and microcrystalline cellulose. The tablet coating film consists of FD&C Blue No. 1, FD&C Yellow No. 5 (tartrazine), ferric oxide yellow, hypromellose 2910, polydextrose, polyethylene glycol 8000, titanium dioxide and triacetin. Distributed by: TG Therapeutics, Inc., Edison, NJ 08837 UKONIQ™ is a trademark of TG Therapeutics, Inc. © TG Therapeutics, Inc. 2020. For more information, go to www.ukoniq.com or call 1-877-848-9462. |
||
| UKONIQ
umbralisib tablet, film coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - TG Therapeutics, Inc. (117372682) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alembic Pharmaceuticals Limited | 650574671 | manufacture(73150-200) | |