Label: HEPAGAM B (hepatitis b immune globulin intravenous- human injection
- NDC Code(s): 70257-051-05, 70257-051-51, 70257-052-11, 70257-052-51
- Packager: Saol Therapeutics Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated March 23, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HepaGam B® safely and effectively. See full prescribing information for HepaGam B®.
HepaGam B® [Hepatitis B Immune Globulin Intravenous (Human)], Sterile Solution for Intravenous or Intramuscular Injection Solvent/Detergent Treated and Filtered. greater than 312 international units per milliliter (Measured potency stamped on the vial label)
Initial U.S. Approval: 2006
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
- For Intravenous or Intramuscular Administration Only
- Prevention of Hepatitis B recurrence following Liver Transplantation in HBsAg-positive liver transplant patients (1.1).
- Postexposure Prophylaxis (1.2) in the following settings:
- Acute Exposure to Blood Containing HBsAg
- Perinatal Exposure of Infants Born to HBsAg-positive Mothers
- Sexual Exposure to HBsAg-positive Persons
- Household Exposure to Persons with Acute HBV Infection
DOSAGE AND ADMINISTRATION
Prevention of Hepatitis B recurrence following liver transplantation (2.1)
HepaGam B is administered intravenously at doses of 20,000 international units (calculated from the measured potency stamped on the vial label) according to the following regimen to attain serum anti-HBs greater than 500 international units per liter:
Regularly monitor serum anti-HBs to allow for treatment adjustments.
Anhepatic Phase Week 1 Post-Operative Weeks 2-12 Post-Operative Month 4 onwards First dose Daily from Day 1-7 Every two weeks from Day 14 Monthly Postexposure Prophylaxis (2.2)
HepaGam B must be administered intramuscularly only as directed below:
Acute Exposure to Blood Containing HBsAg 0.06 milliliter per kilogram Administer as soon as possible after exposure and within 24 hours if possible. Perinatal Exposure of Infants Born to HBsAg-positive Mothers 0.5 milliliter Administer after physiologic stabilization of the infant and preferably within 12 hours of birth. Sexual Exposure to HBsAg-positive Persons 0.06 milliliter per kilogram Administer HepaGam B within 14 days of the last sexual contact or if sexual contact with the infected person will continue Household Exposure to Persons with Acute HBV Infection 0.5 milliliter Infants less than 12 months: Administer HepaGam B + Hepatitis B vaccine if primary caregiver has acute HBV infection. DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The only adverse reactions observed in clinical trial subjects were hypotension and nausea (2% of clinical trial subjects). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Saol Therapeutics at 1-833-644-4216 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Efficacy of live attenuated virus vaccines may be impaired by immune globulin administration; revaccination may be necessary. (7.1)
- Antibodies in HepaGam B may interfere with some serological tests. (7.2)
- Maltose in HepaGam B may interfere with non-glucose specific blood glucose testing systems. (7.3)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Prevention of Hepatitis B recurrence following liver transplant in HBsAg-positive liver transplant patients
1.2 Post-exposure prophylaxis
2 DOSAGE AND ADMINISTRATION
2.1 Prevention of Hepatitis B recurrence following liver transplantation
2.2 Postexposure Prophylaxis
2.3 Preparation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Interference with Blood Glucose Testing
5.3 Monitoring: Serum Anti-HBs Antibody Levels
5.4 Infusion Reactions
5.5 Transmissible Infectious Agents
5.6 Coagulation Disorders
5.7 Thrombotic Events
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Live Attenuated Virus Vaccines
7.2 Drug-Laboratory Interactions: Serological Testing
7.3 Drug-Laboratory Interactions: Blood Glucose Testing
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacokinetics
14 CLINICAL STUDIES
14.1 Clinical Trials in Liver Transplant Patients
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
HepaGam B [Hepatitis B immune globulin intravenous (Human)] is an intravenous immune globulin indicated for the following:
1.1 Prevention of Hepatitis B recurrence following liver transplant in HBsAg-positive liver transplant patients
1.2 Post-exposure prophylaxis
including
- acute exposure to HBsAg-positive blood, plasma, or serum (parenteral exposure, direct mucus membrane contact, oral ingestion, etc.),
- perinatal exposure of infants born to HBsAg-positive mothers,
- sexual exposure to HBsAg-positive persons, and
- household exposure to persons with acute HBV infection.
-
2 DOSAGE AND ADMINISTRATION
2.1 Prevention of Hepatitis B recurrence following liver transplantation
Administer the first dose of HepaGam B during the grafting of the transplanted liver (the anhepatic phase) with subsequent dosing as recommended in Table 1.
Calculate the dosing from the measured potency of the particular lot of HepaGam B as stamped on the vial label.
Administer by intravenous infusion (Table 2).
Table 1 - HepaGam B Dosing Regimen for HBV-Related Liver Transplant Patients * Each dose should contain 20,000 international units calculated from the measured potency as stamped on the vial label [see Dosage Forms and Strengths (3)].
Anhepatic Phase Week 1 Post-Operative Weeks 2-12 Post-Operative Month 4 onwards First dose Daily from Day 1-7 Every two weeks from Day 14 Monthly Table 2 – HepaGam B Intravenous Infusion Rate Route of Administration Dosage Infusion Rate Intravenous 20,000 international units per dose 2 milliliters per minute.
Decrease to 1 milliliter per minute or slower if the patient develops discomfort or infusion-related adverse reactions.HepaGam B dose adjustments may be required in patients who fail to reach anti-HBs levels of 500 International Units per liter within the first week post-liver transplantation1. Patients who have surgical bleeding or abdominal fluid drainage (greater than 500 milliliters) or patients who undergo plasmapheresis are particularly susceptible to extensive loss of circulated anti-HBs. In these cases, the dosing regimen should be increased to a half-dose (10,000 International Units calculated from the measured potency as stamped on the vial label) intravenously every 6 hours until the target anti-HBs is reached.
2.2 Postexposure Prophylaxis
Administer HepaGam B intramuscularly as recommended in Table 3.
Table 3 – HepaGam B Dosing Regimen for Postexposure Prophylaxis (Intramuscular) Indication Dosage Instructions Acute Exposure to Blood Containing HBsAg 0.06 milliliter per kilogram Administer HepaGam B as soon as possible after exposure. The value after seven days following exposure is unclear2, 3.For persons who refuse Hepatitis B vaccine or who are known non-responders to vaccine, give a second dose of HepaGam B one month after the first dose2. Perinatal exposure of Infants Born to HBsAg-positive mothers 0.5 milliliter Administer after physiologic stabilization of the infant and preferably within twelve hours of birth. Administer concurrently with Hepatitis B vaccine. Sexual Exposure to HBsAg-Positive Persons 0.06 milliliter per kilogram Administer HepaGam B and Hepatitis B Vaccine series within 14 days of sexual contact or if sexual contact with the infected person will continue. Household Exposure to Person with Acute HBV Infection 0.5 milliliter For infants less than twelve months of age administered concurrently with Hepatitis B Vaccine. Prophylaxis of other household contacts of persons with acute HBV infection is not indicated unless there is an identifiable blood exposure to the index patient, such as by sharing toothbrushes or razors. Treat such exposures like sexual exposures. HepaGam B may be administered at the same time (but at a different site), or up to one month preceding Hepatitis B vaccination without impairing the active immune response to Hepatitis B vaccine2,3.
2.3 Preparation
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if turbid.
- Do not shake vials during preparation to avoid foaming.
- The HepaGam B vial is for single use only. HepaGam B contains no preservatives.
- Promptly use any vial of HepaGam B that has been entered. Do not reuse or save for future use.
- For intravenous administration, administer HepaGam B through a separate intravenous line using an infusion pump.
- Use normal saline as the diluent if dilution of HepaGam B is preferred prior to intravenous administration. [see Clinical Trials in Liver Transplant Patients (14.1)]
- Do not use dextrose (5%) in water (D5W).
- Use a separate vial, sterile syringe, and needle for each individual patient, to prevent transmission of infectious agents from one person to another.
-
3 DOSAGE FORMS AND STRENGTHS
- HepaGam B is a sterile solution of purified gamma globulin (5% or 50 milligrams per milliliter) that contains greater than 312 International Units per milliliter of anti-HBs.
- The measured potency of each lot is stamped on the vial label.
- To ensure that the label claim of greater than 312 International Units per milliliter is maintained over the product shelf life, a higher potency of 550 International Units per milliliter is targeted at the time of manufacture.
- This higher target potency is a manufacturing requirement to account for variability in the potency assay and changes in potency over time.
- The potency assay has a relative standard deviation (RSD) of approximately 10%.
- The actual potency test result may vary from approximately 400 to 700 International Units per milliliter (3x RSD) based on statistical assessment of manufactured lots with a target potency of 550 International Units per milliliter.
- Calculate the dosing for the prevention of hepatitis B recurrence following liver transplantation from the measured potency of the particular lot of HepaGam B as stamped on the vial label.
-
4 CONTRAINDICATIONS
- Individuals known to have anaphylactic or severe systemic reactions to the parenteral administration of human globulin preparations should not receive HepaGam B.
- Individuals who are deficient in IgA may have the potential to develop anti-IgA antibodies and have an anaphylactoid reaction.
- HepaGam B contains less than 40 micrograms per milliliter of IgA.
- For postexposure prophylaxis indications, HepaGam B must be administered intramuscularly only. In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, HepaGam B should be given only if the expected benefits outweigh the potential risks.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur with HepaGam B. HepaGam B should be administered in a setting with appropriate equipment, medication and personnel trained in the management of hypersensitivity, anaphylaxis and shock. In case of hypersensitivity, discontinue HepaGam B infusion immediately and begin appropriate emergency treatment. Medications such as epinephrine and antihistamines should be available for immediate treatment of acute hypersensitivity reactions.
HepaGam B contains trace amounts of IgA (less than 40 micrograms per milliliter). Patients with known antibodies to IgA may have a greater risk of severe hypersensitivity and anaphylactic reactions. HepaGam B is contraindicated in IgA deficient patients with antibodies against IgA and history of hypersensitivity reaction. (see CONTRAINDICATIONS [4])
5.2 Interference with Blood Glucose Testing
The maltose contained in HepaGam B can interfere with some types of blood glucose monitoring systems, i.e., those based on the glucose dehydrogenase pyrroloquine quinone (GDH-PQQ) method. This can result in falsely elevated glucose readings and, consequently, in the inappropriate administration of insulin, resulting in life-threatening hypoglycemia. Cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated results.
5.3 Monitoring: Serum Anti-HBs Antibody Levels
Liver transplant patients should be monitored regularly for serum anti-HBs antibody levels using a quantitative assay to ensure that adequate protective levels are maintained.
5.4 Infusion Reactions
Certain adverse drug reactions may be related to the rate of infusion. The recommended infusion rate given under Dosage and Administration (2.1) must be closely followed. Patients must be closely monitored and carefully observed for any symptoms throughout the infusion period and immediately following an infusion.
5.5 Transmissible Infectious Agents
Because HepaGam B is made from human plasma, it may carry a risk of transmitting infectious agents, e.g. viruses, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. No cases of transmission of viral diseases or CJD have been associated with the use of HepaGam B. All infections suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Saol Therapeutics at 1-833-644-4216.
5.6 Coagulation Disorders
For postexposure prophylaxis indications, HepaGam B must be administered intramuscularly only. In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, HepaGam B should be given only if the expected benefits outweigh the potential risks.
5.7 Thrombotic Events
Thrombotic events may occur during or following treatment with IGIV products4, 5. Patients at risk include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization, and/or known/suspected hyperviscosity.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity including those with cryoglobulins, fasting
chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients who are at risk of developing thrombotic events, administer HepaGam B at the minimum rate of infusion practicable. -
6 ADVERSE REACTIONS
The only adverse reactions observed in clinical trial subjects were hypotension and nausea (2% of clinical trial subjects).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hepatitis B-Related Liver Transplantation
In a clinical trial with 27 liver transplant patients, one adverse drug reaction was reported following the 578 (less than 1%) HepaGam B infusions. This study utilized the recommended dosing regimen outlined in Table 1 [see Dosage and Administration (2.1)]. The attributed adverse drug reaction of hypotension was reported in one patient. The reaction was associated with a single HepaGam B infusion during the first day post-transplant. The reaction resolved on the same day and did not recur with subsequent HepaGam B infusions.Healthy Volunteer Studies
Seventy healthy male and female volunteers received a single dose of HepaGam B intramuscularly in clinical trials6. Only one adverse drug reaction, an episode of nausea, was reported.6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of HepaGam B. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postexposure Prophylaxis:
Dizziness has been reported in the postmarketing surveillance of HepaGam B for the postexposure prophylaxis indication.
Hepatitis B-Related Liver Transplantation:
The system organ classification of reported adverse reactions is provided below:
Cardiac disorders:
Sinus tachycardia
Gastrointestinal disorders:
Abdominal pain upper
Nausea
General disorders and administration site conditions:
Chills
Feeling cold
Influenza like illness
Pyrexia
Immune system disorders:
Anaphylactoid reaction
Hypersensitivity
Investigations:
Lipase increased
Transaminases increased
Musculoskeletal and connective tissue disorders:
Back pain
Nervous system disorders:
Dizziness
Headache
Respiratory, thoracic and mediastinal disorders:
Dyspnoea
Skin and subcutaneous tissue disorders:
Cold sweat
Healthcare professionals should report adverse reactions following the administration of HepaGam B to Saol Therapeutics at 1-833-644-4216 or FDA’s MedWatch reporting system at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
7 DRUG INTERACTIONS
7.1 Live Attenuated Virus Vaccines
Immune globulin administration may impair the efficacy of live attenuated virus vaccines such as measles, rubella, mumps and varicella2,3,7. Vaccination with live virus vaccines should be deferred until approximately three months after administration of HepaGam B, Hepatitis B Immune Globulin Intravenous (Human). Persons who received HepaGam B less than 14 days after live virus vaccination should be revaccinated 3 months after the administration of the immune globulin, unless serologic test results indicate that antibodies were produced2,3.
There are no available data on drug interactions of HepaGam B with other medications.
7.2 Drug-Laboratory Interactions: Serological Testing
Antibodies present in HepaGam B may interfere with some serological tests. After administration of immune globulins like HepaGam B, a transitory increase of passively transferred antibodies in the patient’s blood may result in misleading positive results in serological testing (e.g. Coombs' test).
7.3 Drug-Laboratory Interactions: Blood Glucose Testing
HepaGam B contains maltose which can interfere with certain types of blood glucose monitoring systems. [See Warnings and Precautions (5.2).] Only testing systems that are glucose-specific should be used in patients receiving HepaGam B. This interference can result in falsely elevated glucose readings that can lead to untreated hypoglycemia or to inappropriate insulin administration, resulting in life-threatening hypoglycemia. The product information of the blood glucose testing system, including that of the test strips, should be carefully reviewed to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, contact the manufacturer of the testing system to determine if the system is appropriate for use with maltose-containing parenteral products.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with HepaGam B. It is also not known whether HepaGam B can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. HepaGam B should be given to a pregnant woman only if clearly indicated.
8.2 Lactation
It is not known whether HepaGam B is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when HepaGam B is administered to a nursing mother.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients. However, for postexposure prophylaxis, the safety and effectiveness of similar hepatitis B immune globulins have been demonstrated in infants and children8.
8.5 Geriatric Use
Clinical studies of HepaGam B did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- 10 OVERDOSAGE
-
11 DESCRIPTION
HepaGam B, Hepatitis B Immune Globulin Intravenous (Human), is a solvent/detergent-treated sterile solution of purified gamma globulin containing anti-HBs. It is prepared from plasma donated by healthy, screened donors with high titers of anti-HBs that is purified by an anion-exchange column chromatography manufacturing method9,10. HepaGam B is formulated as a 5% (50 milligrams per milliliter) protein solution with 10% maltose and 0.03% polysorbate 80 at pH 5.6. It is available in 1 milliliter and 5 milliliters single dose vials. The product appears as a clear to opalescent liquid. HepaGam B does not contain mercury. It contains no preservatives. This product is intended for single use. HepaGam B may be administered intravenously or intramuscularly dependent upon indication [see Dosage and Administration (2.)]. The source plasma used in the manufacture of this product was tested by FDA licensed Nucleic Acid testing (NAT) for HIV-1, HBV and HCV and found to be negative. Plasma also has been tested by in-process NAT for hepatitis A virus (HAV) and parvovirus B19 (B19) via minipool testing and the limit for B19 in the manufacturing pool is set not to exceed 104 international units of B19 DNA per milliliter.
The manufacturing process contains two steps implemented specifically for virus clearance. The solvent and detergent step (using tri-n-butyl phosphate and Triton® X-100) is effective in the inactivation of enveloped viruses, such as hepatitis B, hepatitis C and HIV11. Virus filtration, using a Planova® 20N virus filter, is effective for the removal of viruses based on their size, including some non-enveloped viruses12. These two viral clearance steps are designed to increase product safety by reducing the risk of transmission of enveloped and non-enveloped viruses. In addition to these two specific steps, the process step of anion-exchange chromatography was identified as contributing to the overall viral clearance capacity for small non-enveloped viruses.
The inactivation and reduction of known enveloped and non–enveloped model viruses were validated in laboratory studies as summarized in Table 4. The viruses employed for spiking studies were selected to represent those viruses that are potential contaminants in the product, and to represent a wide range of physiochemical properties in order to challenge the manufacturing process’s ability for viral clearance in general.
Table 4 - Virus Reduction Values Obtained Through Validation Studies8 Abbreviations:
HIV-1: human immunodeficiency virus-1; relevant virus for human immunodeficiency virus-1 and model for HIV-2
BVDV: bovine viral diarrhea virus; model virus for hepatitis C virus (HCV) and West Nile virus (WNV)
PRV: pseudorabies virus; model for large enveloped DNA viruses, including herpes
HAV: human hepatitis A virus; relevant virus for HAV and model for small non-enveloped viruses in general
EMC: encephalomyocarditis virus; model for HAV and for small non-enveloped viruses in general
MMV: murine minute virus; model for human parvovirus B19 and for small non-enveloped viruses in general
PPV: porcine parvovirus; model for human parvovirus B19 and for small non-enveloped viruses in general
n.e.: not evaluated
≥: greater than or equal to
a The PRV was retained by the 0.1 µm pre-filter during the virus validation. Since manufacturing employs a 0.1 µm pre-filter before the 20N filter, the claim of greater than equal to 5.6 reduction is considered applicable.
Enveloped Non-Enveloped Genome RNA DNA RNA DNA Virus HIV-1 BVDV PRV HAV EMC MMV PPV Family retro flavi herpes picorna parvo Size (nm) 80-100 50-70 120-200 25-30 30 20-25 18-24 Anion Exchange Chromatography (partitioning) Not evaluated 2.3 n.e. 3.4 n.e. 20N Filtration (size exclusion) ≥4.7 ≥3.5 ≥5.6a n.e. 4.8 n.e. 4.1 Solvent/Detergent (inactivation) ≥4.7 ≥7.3 ≥5.5 Not evaluated Total Reduction (log10) ≥9.4 ≥10.8 ≥11.1 2.3 4.8 3.4 4.1 The product potency is expressed in international units by comparison to the World Health Organization (WHO) standard Hepatitis B Immune Globulin. Each vial contains greater than 312 international units per milliliter. The measured potency of each lot is stamped on the vial label [see Dosage Forms and Strengths (3)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
HepaGam B provides passive immunization for individuals exposed to the hepatitis B virus, by binding to the surface antigen and reducing the rate of hepatitis B infection13-16.
12.2 Pharmacokinetics
The pharmacokinetic profile of HepaGam B has been evaluated in two 84-day clinical trials in which 70 healthy subjects received an intramuscular injection of 0.06 milliliter per kilogram of HepaGam B. The mean peak concentrations (Cmax) in both studies were comparable and occurred within 4-5 days of administration. Both studies demonstrated mean elimination half-lives (t½) following IM administration of 22 to 25 days. The mean clearance rate was 0.21 to 0.24 liter per day and the volume of distribution was approximately 7.5 liter. Thus, HepaGam B demonstrates pharmacokinetic parameters similar to those reported by Scheiermann and Kuwert17.
The maximum concentration of anti-HBs achieved by HepaGam B was consistent with that of two other licensed comparator Hepatitis B Immune Globulin (Human) products 6. Comparability of pharmacokinetics between HepaGam B and a commercially available hepatitis B immune globulin product administered IM indicates that comparable efficacy of HepaGam B should be inferred.
-
14 CLINICAL STUDIES
14.1 Clinical Trials in Liver Transplant Patients
A clinical trial examined the effectiveness of HepaGam B in the prevention of hepatitis B recurrence following liver transplantation. The study was a multi-center, open-labeled, superiority study involving HBsAg-positive/HBeAg-negative liver transplant patients. The study included two arms, an active treatment group of patients enrolled to receive the described dosing regimen of HepaGam B starting during transplant and continuing over the course of a year, and a retrospective untreated control group of historical patients with data gathered by chart review.
There were 27 liver transplant patients who received HepaGam B and 14 retrospective untreated control patients. The patients in both groups were HBsAg-positive/HBeAg-negative liver transplant patients who met similar entry criteria, had similar medical history and had similar status at transplant based on MELD and/or ChildPugh-Turcotte scores.
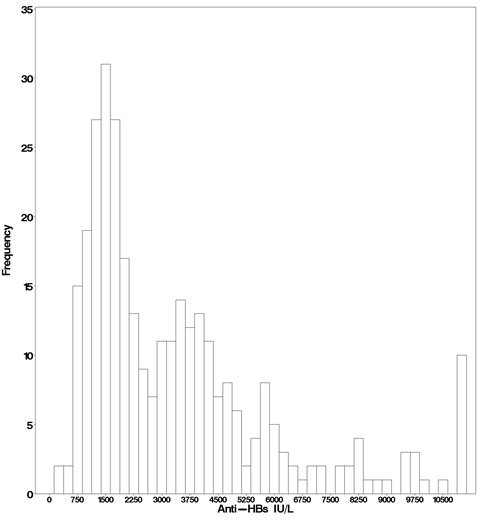
In the active treatment group, HepaGam B intravenous doses of 35 milliliters were initiated during transplant according to the regimen identified in Table 1 [see Dosage and Administration (2.1)]. As a result of the targeted potency of 550 international units per milliliter at the time of manufacture [see Dosage Forms and Strengths (3.)], the 35 milliliter doses of HepaGam B used in this study actually contained between 17,000 and 23,000 international units anti-HBs. These 35 milliliter doses consistently yielded anti-HBs trough levels greater than 500 international units per liter (99% of all anti-HBs levels were greater than 500 international units per liter; see Figure 1). Patients received HepaGam B doses diluted with 50 mL of saline.
Figure 1: Frequency Histogram of Trough anti-HBs Levels more than 30 days after Transplant
Values below the target trough were only observed in the 2 patients with HBV recurrence who had anti-HBs levels less than 150 international units per liter at the time of seroconversion.
For the efficacy endpoint of the proportion of patients with HBV recurrence (HBsAg positive and/or HBeAg positive after 4 weeks post-OLT), a significant treatment effect was observed. As summarized in Table 5, HBV recurrence was seen in 2/24 or 8.3% of HepaGam B patients compared to 12/14 or 86% of retrospective untreated control patients (see Table 5). Two of the HepaGam B patients who died within 28 days post-transplant were excluded from all efficacy analyses, but included for safety analyses. The deaths were not HBV or study drug related.
Table 5 - Results of Study HB-005 for the Prevention of Hepatitis B Recurrence Following Liver Transplantation HepaGam B Retrospective Untreated Control P-value (Fisher's Exact Test) HBV Recurrence
Proportion, % (95% confidence interval)8.3
(0.1 -27.0)85.7
(57.2 -98.2)less than 0.001 The conclusion that HepaGam B monotherapy post-OLT is effective at preventing HBV recurrence post-OLT is further supported by the secondary endpoints of time to recurrence, survival, anti-HBs levels, biochemical markers of liver inflammation, and liver biopsy. Time to recurrence for the HepaGam B treatment group was 358 days for two HBV recurrent patients. In comparison, the retrospective untreated control patients had a median time to recurrence of 88 days with a 95% confidence interval of 47 to 125 days. Survival calculations showed that 96% (23/24) of patients in the active treatment group survived for at least 1 year post-OLT compared to 43% (6/14) retrospective control patients. The endpoints for HBV recurrence were supported by an observed drop in anti-HBs levels, elevated liver function tests, and abnormal liver biopsy result at the time of recurrence.
HepaGam B is recommended in patients who have no or low levels of viral replication at the time of liver transplantation. The clinical trial evaluating HepaGam B in liver transplant patients selected patients with no or low replication status only. HepaGam B therapy has not been evaluated in combination with antiviral therapy post-transplantation.
-
15 REFERENCES
- McGory RW, Ishitani MB, Oliveira WM, Stevenson WC, McCullough CS, Dickson RC et al. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation 1996; 61(9):1358-1364.
- CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: Immunization of infants, children, and adolescents. MMWR 2005; 54(RR-16): 1-32.
- CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 2: Immunization of adults. MMWR 2006; 55(RR-16): 1-33.
- Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994; 44:223-226.
- Woodruff RK, et al.: Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986; 2:217-218.
- Unpublished data on file.
- Committee for Proprietary Medicinal Products (CPMP). Core SPC for human plasma derived hepatitis-B immunoglobulin for intravenous use (CPMP/BPWG/4027/02). London, UK: The European Agency for the Evaluation of Medicinal Products. 2003.
- CDC: Recommendations for protection against viral hepatitis. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR 1985; 34(22):313-335.
- Bowman JM, et al. WinRho: Rh immune globulin prepared by ion exchange for intravenous use. Canadian Med Assoc J 1980; 123:1121-5.
- Friesen AD, et al. Column ion-exchange preparation and characterization of an Rh immune globulin (WinRho) for intravenous use. Journal Appl Biochem 1981; 3:164-75.
- Horowitz B. Investigations into the application of tri(n-butyl)phosphate /detergent mixtures to blood derivatives. Morgenthaler J (ed): Virus Inactivation in Plasma Products, Curr Stud Hematol Blood Transfus 1989; 56:83-96.
- Burnouf T. Value of virus filtration as method for improving the safety of plasma products. Vox Sang 1996; 70:235-6.
- Grady GF, Lee VA. Hepatitis B immune globulin - prevention of hepatitis from accidental exposure among medical personnel. N Engl J Med 1975; 293:1067-70.
- Seeff LB, et al. Type B hepatitis after needle-stick exposure: Prevention with hepatitis B immune globulin. Ann Int Med 1978; 88: 285-93.
- Krugman S, Giles JP. Viral hepatitis, type B (MS-2-strain). Further observations on natural history and prevention. N Engl J Med 1973; 288: 755-60.
- Hoofnagle JH, et al. Passive-active immunity from hepatitis B immune globulin. Ann Int Med 1979; 91:813-8.
- Scheiermann N, Kuwert EK. Uptake and elimination of hepatitis B immunoglobulins after intramuscular application in man. Dev Biol Standard 1983; 54:347-55.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC 70257-052-51; a carton containing a 1.0 milliliter single dose vial (greater than 312 international units per milliliter; measured potency of each lot is stamped on the vial label) and a package insert.
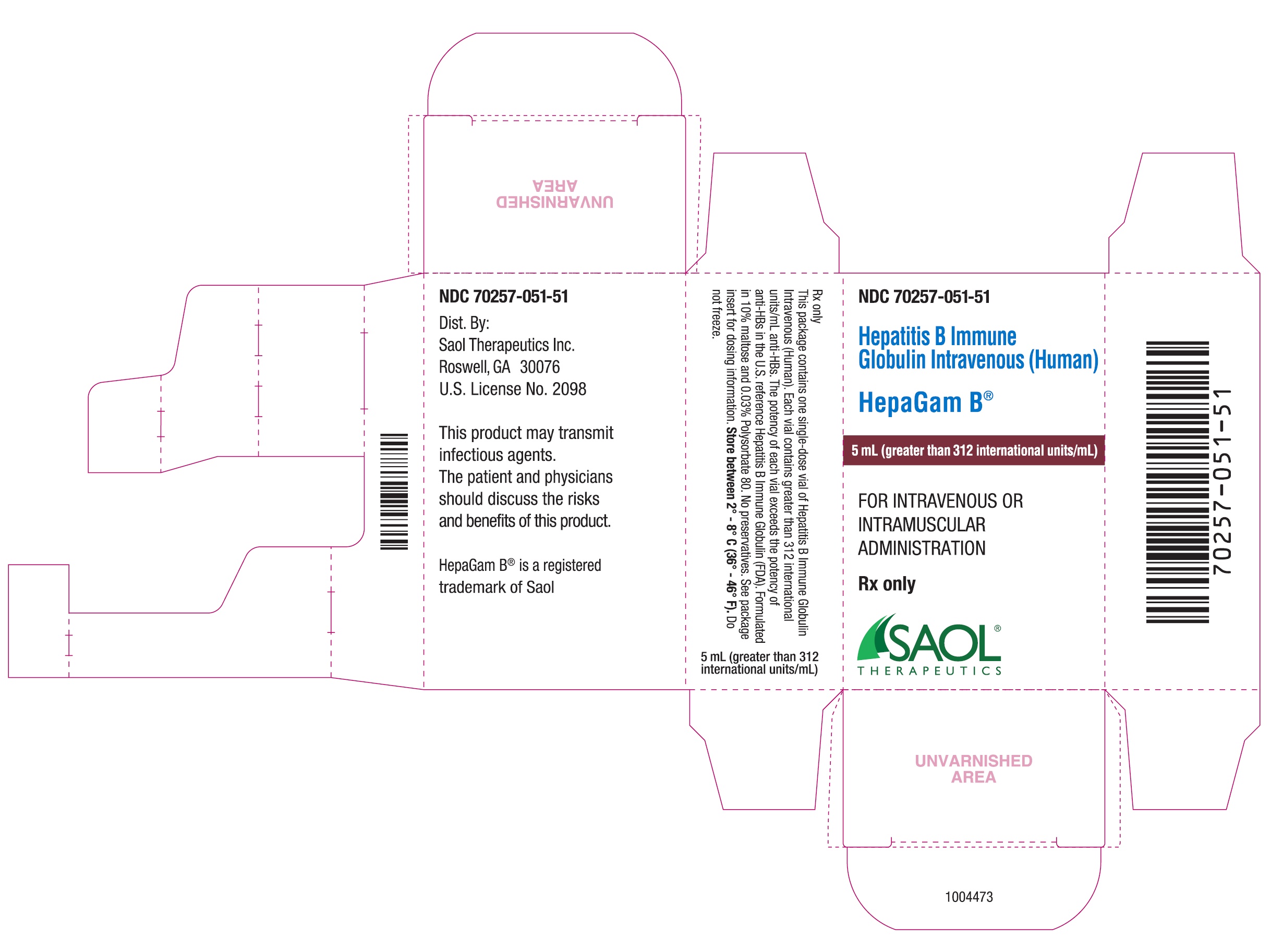
NDC 70257-051-51; a carton containing a 5.0 milliliter single dose vial (greater than 312 international units per milliliter; measure potency of each lot is stamped on the vial label) and a package insert.
Store at 36 to 46 °F (2 to 8 °C). Do not freeze. Do not use after expiration date. Use within 6 hours after the vial has been entered.
-
17 PATIENT COUNSELING INFORMATION
- Inform patients of the following:
- HepaGam B is prepared from human plasma, and therefore, may contain infectious agents such as viruses that can cause disease.
- The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses during manufacturing.
- Despite these measures, such products can still potentially transmit disease.
- There is also the possibility that unknown infectious agents may be present in such products.
- Tell patients that persons known to have severe, potentially life-threatening reactions to human globulin products should not receive HepaGam B or any other immune globulin products unless the risk has been justified.
- Tell patients that persons who are deficient in IgA may have the potential for developing anti-IgA antibodies and have severe potentially life threatening allergic reactions.
- In case of allergic or anaphylactic reaction, the infusion should be stopped immediately.
- In case of shock, the current medical standards for treatment of shock should be observed.
- Advise Liver Transplant patients about the potential interference with non-glucose specific monitoring systems.
- The maltose contained in HepaGam B can interfere with some types of blood glucose monitoring systems.
- Only testing systems that are glucose-specific should be used in patients receiving HepaGam B.
- This inference can result in falsely elevated glucose readings that can lead to untreated hypoglycemia or to inappropriate insulin administration, resulting in life-threatening hypoglycemia.
HepaGam B® [Hepatitis B Immune Globulin Intravenous (Human)] Sterile Solution for Injection and any and all Saol brand, product, service and feature names, logos, slogans are trademarks or registered trademarks of Saol. All rights reserved.
PLANOVA® is a registered trademark of Asahi Kasei Medical Co., Ltd, TRITON® is a registered trademark of Union Carbide Corporation.
Distributed by:
Saol Therapeutics Inc.
Roswell, GA 30076
U.S. License No. 2098
- Inform patients of the following:
- PRINCIPAL DISPLAY PANEL - NDC: 70257-052-11 - 1 mL Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-052-51 - 1 mL Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-051-05 - 5 mL Vial Label
- PPRINCIPAL DISPLAY PANEL - NDC: 70257-051-51 - 5 mL Carton Label
-
INGREDIENTS AND APPEARANCE
HEPAGAM B
hepatitis b immune globulin intravenous (human) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70257-052 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN HEPATITIS B VIRUS IMMUNE GLOBULIN (UNII: XII270YC6M) (HUMAN HEPATITIS B VIRUS IMMUNE GLOBULIN - UNII:XII270YC6M) HUMAN HEPATITIS B VIRUS IMMUNE GLOBULIN 312 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MALTOSE (UNII: XJ6S9RV06F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70257-052-51 1 in 1 CARTON 03/01/2019 1 NDC:70257-052-11 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125035 03/01/2019 HEPAGAM B
hepatitis b immune globulin intravenous (human) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70257-051 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN HEPATITIS B VIRUS IMMUNE GLOBULIN (UNII: XII270YC6M) (HUMAN HEPATITIS B VIRUS IMMUNE GLOBULIN - UNII:XII270YC6M) HUMAN HEPATITIS B VIRUS IMMUNE GLOBULIN 312 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength MALTOSE (UNII: XJ6S9RV06F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70257-051-51 1 in 1 CARTON 03/01/2019 1 NDC:70257-051-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125035 03/01/2019 Labeler - Saol Therapeutics Inc. (080040201)