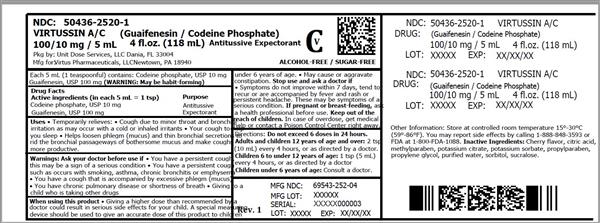
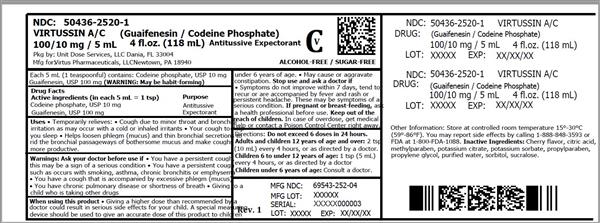
Label: VIRTUSSIN A/C- codeine phosphate and guaifenesin liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 50436-2520-1 - Packager: Unit Dose Services
- This is a repackaged label.
- Source NDC Code(s): 69543-252
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: CV
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 8, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Ask your doctor before use if
- you have a persistent cough, this may be a sign of a serious condition
- you have a persistent cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- you have a cough that is accompanied by excessive phlegm (mucus)
- you have chronic pulmonary disease or shortness of breath
- giving to a child who is taking other drugs
When using this product
- giving a higher dose than recommended by a doctor could result in serious side effects for your child. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age.
- may cause or aggravate constipation
- Directions
- Other information
- Inactive ingredients
- HOW SUPPLIED
- VIRTUSSIN A/C (CODEINE PHOSPHATE AND GUAIFENESIN) LIQUID
-
INGREDIENTS AND APPEARANCE
VIRTUSSIN A/C
codeine phosphate and guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50436-2520(NDC:69543-252) Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength citric acid monohydrate (UNII: 2968PHW8QP) methylparaben (UNII: A2I8C7HI9T) potassium citrate (UNII: EE90ONI6FF) potassium sorbate (UNII: 1VPU26JZZ4) propylparaben (UNII: Z8IX2SC1OH) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) sorbitol (UNII: 506T60A25R) sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50436-2520-1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/08/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 07/07/2015 Labeler - Unit Dose Services (831995316) Establishment Name Address ID/FEI Business Operations Unit Dose Services 831995316 REPACK(50436-2520) , RELABEL(50436-2520)