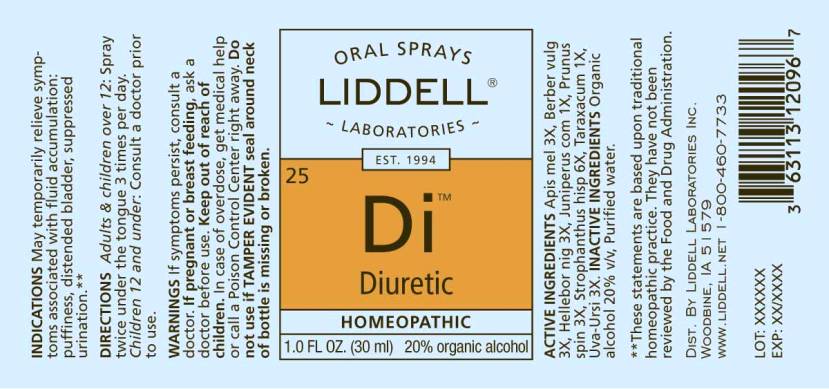
DIURETIC- apis mellifica, berberis vulgaris, helleborus niger, juniperus communis, prunus spinosa, strophanthus hispidus, taraxacum officinale, uva-ursi spray
Liddell Laboratories, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts
ACTIVE INGREDIENTS:
Apis Mellifica 3X, Berberis Vulgaris 3X, Helleborus Niger 3X, Juniperus Communis 1X, Prunus Spinosa 3X, Strophanthus Hispidus 6X, Taraxacum Officinale 1X, Uva-Ursi 3X
INDICATIONS:
May temporarily relieve symptoms associated with fluid accumulation: puffiness, distended bladdder, suppressed urination.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
If symptoms persist, consult a doctor.
If pregnant or breast feeding, ask a doctor before use.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
DIRECTIONS:
Adults and children over 12: Spray twice under the tongue 3 times per day.
Children 12 and under: Consult a doctor prior to use.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
| DIURETIC
apis mellifica, berberis vulgaris, helleborus niger, juniperus communis, prunus spinosa, strophanthus hispidus, taraxacum officinale, uva-ursi spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Liddell Laboratories, Inc. (832264241) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(50845-0106) , api manufacture(50845-0106) , label(50845-0106) , pack(50845-0106) | |