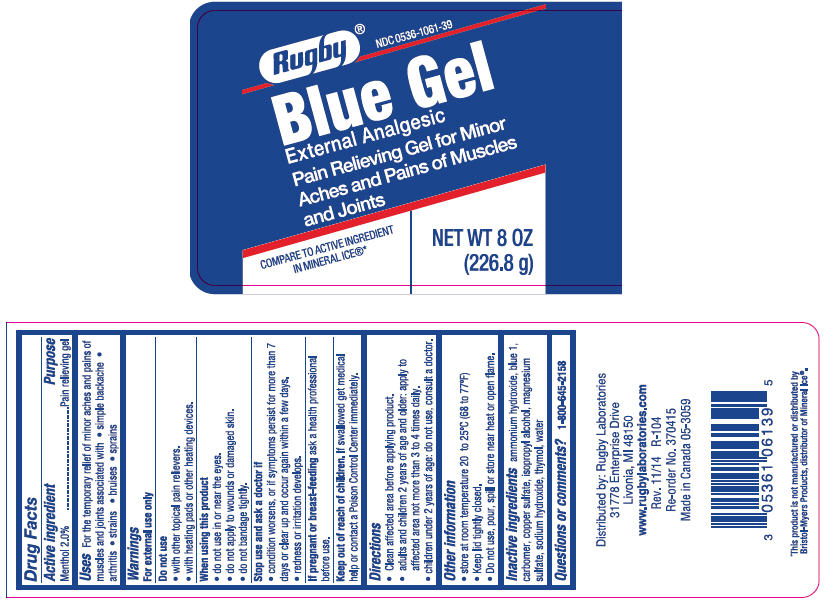
Label: RUGBY ICE BLUE EXTERNAL ANALGESIC- menthol, unspecified form gel
- NDC Code(s): 0536-1061-39
- Packager: Rugby Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- do not use in or near the eyes.
- do not apply to wounds or damaged skin.
- do not bandage tightly.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 226.8 g Jar Label
-
INGREDIENTS AND APPEARANCE
RUGBY ICE BLUE EXTERNAL ANALGESIC
menthol, unspecified form gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 20 mg in 1 g Inactive Ingredients Ingredient Name Strength Ammonia (UNII: 5138Q19F1X) FD&C Blue No. 1 (UNII: H3R47K3TBD) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) Cupric Sulfate (UNII: LRX7AJ16DT) Isopropyl Alcohol (UNII: ND2M416302) Magnesium Sulfate Anhydrous (UNII: ML30MJ2U7I) Sodium Hydroxide (UNII: 55X04QC32I) Thymol (UNII: 3J50XA376E) Water (UNII: 059QF0KO0R) Product Characteristics Color BLUE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1061-39 226.8 g in 1 JAR; Type 0: Not a Combination Product 01/20/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/20/2015 Labeler - Rugby Laboratories, Inc. (079246066) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Garcoa, Inc. 036464697 MANUFACTURE(0536-1061)