HELIUM- helium gas
Air Products and Chemicals, Inc.
----------
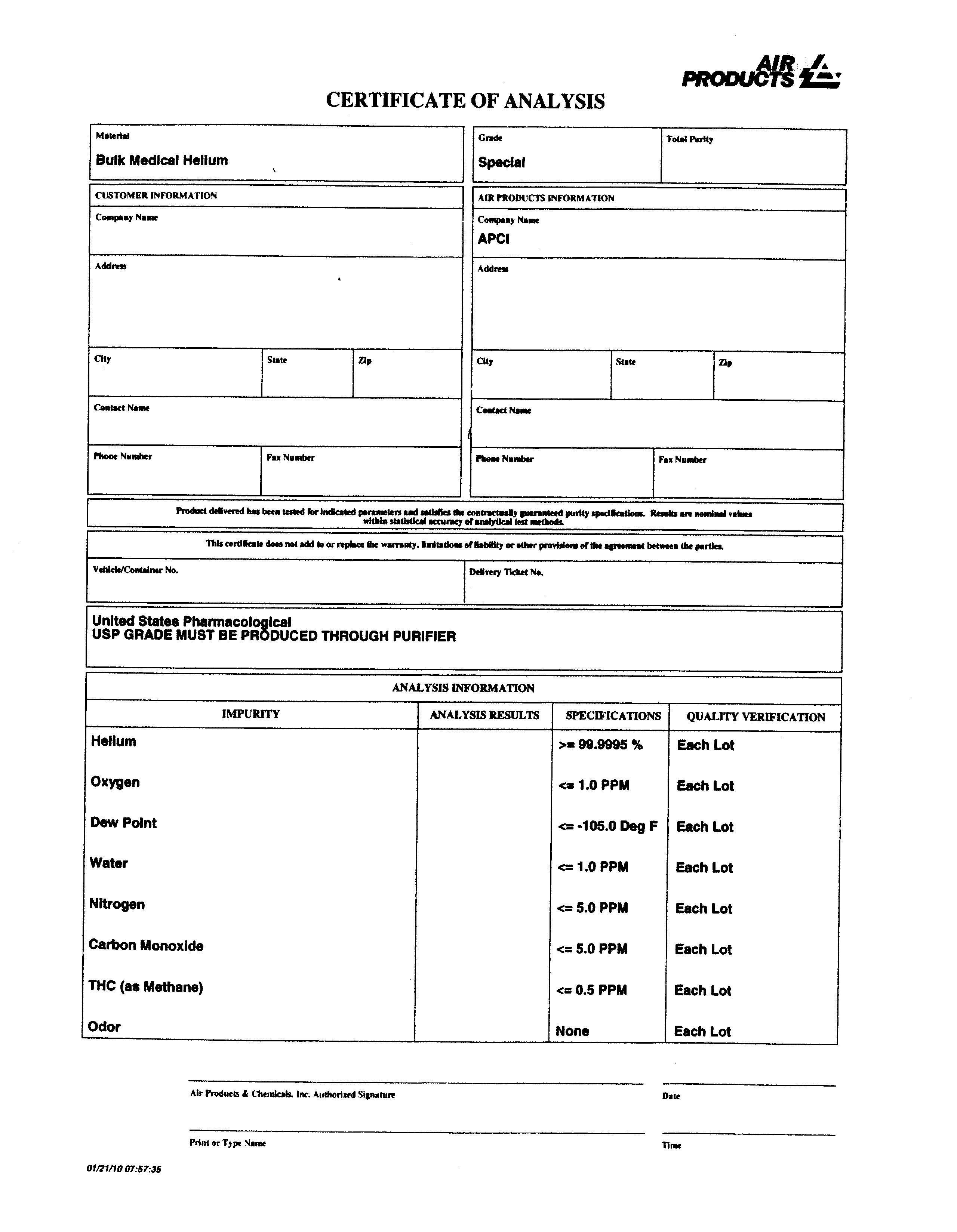
Retire medical Helium
| HELIUM
helium gas |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Air Products and Chemicals, Inc. (003001070) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Air Products and Chemicals, Inc. | 041598673 | manufacture(10018-9505) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Air Products and Chemicals, Inc. | 133211300 | manufacture(10018-9505) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Air Products and Chemicals, Inc. | 134118082 | manufacture(10018-9505) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Air Products and Chemicals, Inc. | 151876851 | manufacture(10018-9505) | |
Revised: 11/2023
Document Id: 091bab7f-9217-8b68-e063-6294a90aac65
Set id: 2b47c44b-9041-40a6-bbf8-fbed6075aeb8
Version: 6
Effective Time: 20231101
Air Products and Chemicals, Inc.