SPF 30 SUNSCREEN TOTTLE W CARABINER- avobenzone, oxybenzone, homosalate, octinoxate, octisalate lotion
INNOVATION SPECIALTIES
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
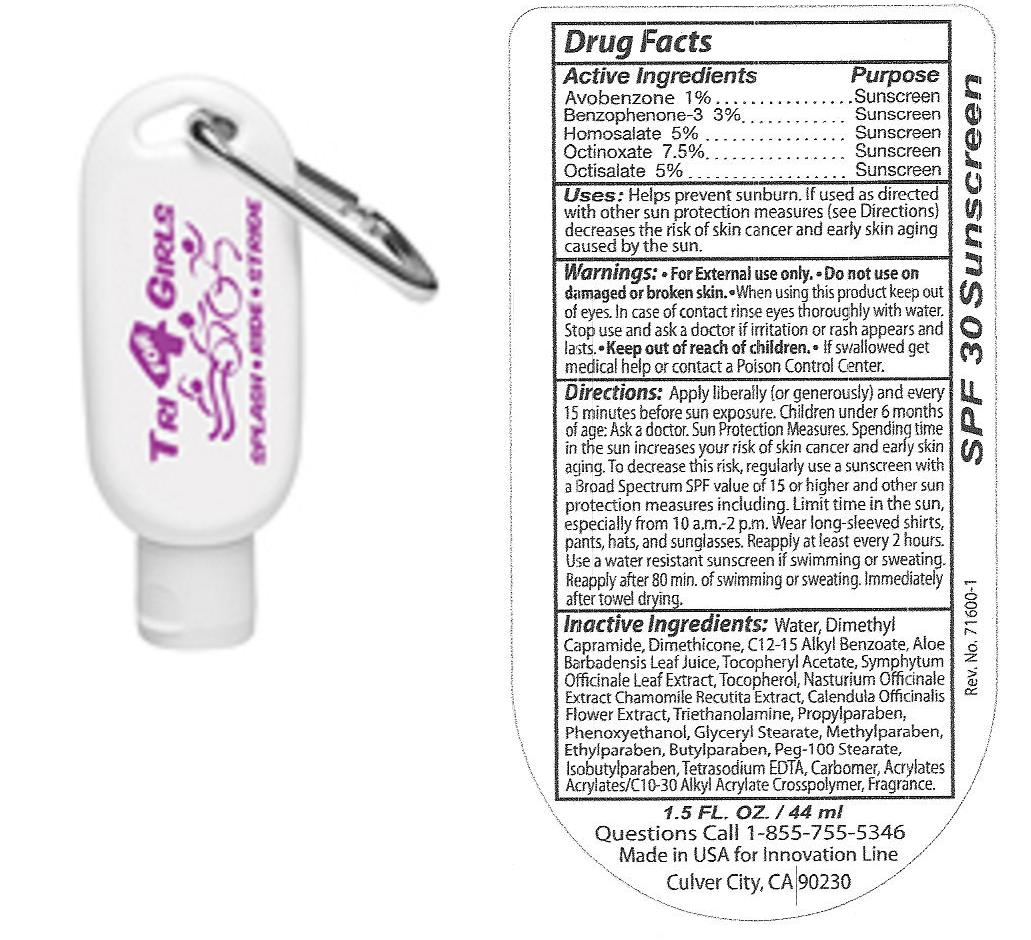
Drug Facts
Directions
Apply liberally (or generously) and every 15 minutes before sun exposure. Children under 6 months of age ask a doctor. Sun Protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including limit time in the sun especially from 10 a.m. - 2 p.m. Wear long-sleeved shirts, pants, hats, and sunglasses. Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating. Reapply after 80 min. of swimming or sweating. Immediately after towel drying.
Warnings:
For external use only. Do not use on damaged or broken skin. when using this product keep out of eyes. In case of contact rinse eyes thoroughly with water. Stop use and ask a doctor if irritation or rash appears and lasts. Keep out of reach of children, if swallowed get medical help or contact a Poison Control Center.
Dosage and Administration
Apply liberally (or generously)15 minutes before sun exposure, and reapply as needed.
Inactive Ingredients
Water, Dimethyl Capramide, Dimethicone, C12-15 Alkyl
Benzoate, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Symphytum Officinale
Leaf Extract, Tocopherol, Nasturium Officinale Extract, Chamomile Recutita
Extract, Calendula Officinalis Flower Extract, Triethanolamine,Propylparaben,Phenoxyethanol, Glyceryl Stearate,
Methylparaben, Ethylparaben, Butylparaben, Peg-100 Stearate, Isobutylparaben,
Tetrasodium Edta, Carbomer, Acrylates, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Fragrance.
| SPF 30 SUNSCREEN TOTTLE W CARABINER
avobenzone, oxybenzone, homosalate, octinoxate, octisalate lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - INNOVATION SPECIALTIES (030837314) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CORETEX PRODUCTS INC | 061944620 | manufacture(76138-202) | |