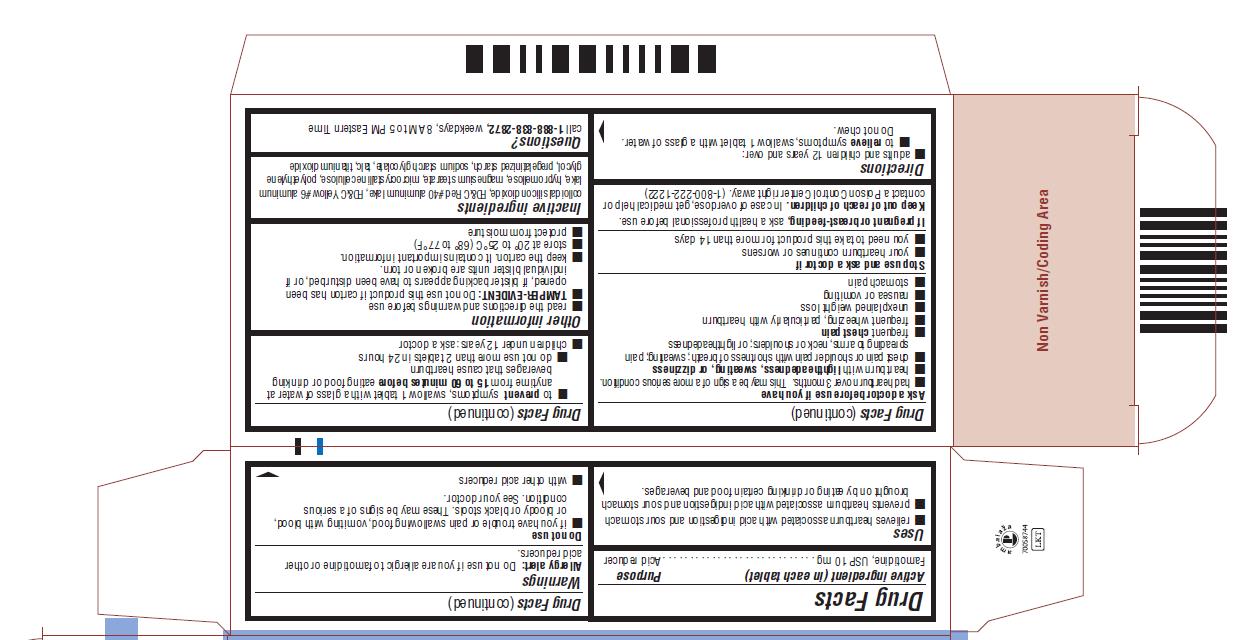
FAMOTIDINE- famotidine tablet, film coated
Teva Pharmaceuticals USA, Inc.
----------
Famotidine
Tablets USP
10 mg
ACID REDUCER
Uses
- relieves heartburn associated with acid indigestion
and sour stomach - prevents heartburn associated with acid indigestion
and sour stomach brought on by eating or drinking
certain food and beverages.
Warnings
Allergy alert: Do not use if you are allergic to famotidine or
other acid reducers.
Do not use
- if you have trouble or pain swallowing food, vomiting
with blood, or bloody or black stools. These may be
signs of a serious condition. See your doctor. - with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a
sign of a more serious condition. - heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath;
sweating; pain spreading to arms, neck or shoulders;
or lightheadedness - frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of
water. Do not chew. - to prevent symptoms, swallow 1 tablet with a glass of
water at anytime from 15 to 60 minutes before eating
food or drinking beverages that cause heartburn - do not use more than 2 tablets in 24 hours
- to relieve symptoms, swallow 1 tablet with a glass of
- children under 12 years: ask a doctor
Other information
- read the directions and warnings before use
-
TAMPER-EVIDENT: Do not use this product if carton has
been opened, if blister backing appears to have been
disturbed, or if individual blister units are broken or torn. - keep the carton. It contains important information.
- store at 20° to 25°C (68° to 77°F)
- protect from moisture
| FAMOTIDINE
famotidine tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Teva Pharmaceuticals USA, Inc. (001627975) |
Revised: 11/2021
Document Id: fdbd1f73-5aa9-46f6-ace8-2742dc8c490e
Set id: 2a8cc600-4995-4279-b169-206d7ef8a93f
Version: 9
Effective Time: 20211116
Teva Pharmaceuticals USA, Inc.