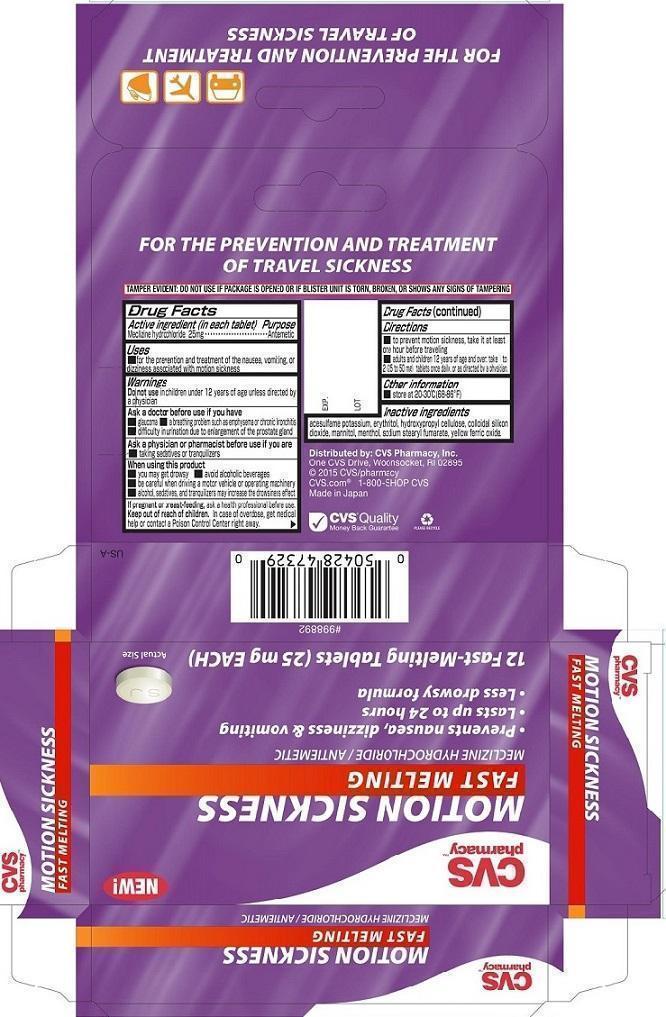
Label: CVS MOTION SICKNESS FAST MELTING- meclizine hydrochloride tablet, orally disintegrating
- NDC Code(s): 59779-534-01
- Packager: CVS Pharmacy, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
Warnings
Ask a doctor before use if you have
■ glaucoma ■ a breathing problem such as emphysema or chronic bronchitis
■ difficulty in urination due to enlargement of the prostate gland - DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS MOTION SICKNESS FAST MELTING
meclizine hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-534 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ERYTHRITOL (UNII: RA96B954X6) MANNITOL (UNII: 3OWL53L36A) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) LEVOMENTHOL (UNII: BZ1R15MTK7) Product Characteristics Color yellow Score no score Shape ROUND Size 13mm Flavor Imprint Code SJ Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-534-01 2 in 1 CARTON 08/27/2015 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 08/27/2015 Labeler - CVS Pharmacy, Inc. (062312574)