KAISER PERMANENTE TERBINAFINE HYDROCHLORIDE- terbinafine hydrochloride cream
Kaiser Foundation Hospitals
----------
KAISER PERMANENTE
®
Terbinafine Hydrochloride
Uses
- cures most athlete's foot (tinea pedis)
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- relieves itching, burning, cracking and scaling which accompany these conditions
Warnings
For external use only
Directions
| 1 week between the toes
 2 weeks on the bottom or sides of the foot 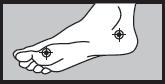 |
Other information
- do not use if seal on tube is broken or is not visible
- store at controlled room temperature 20°-25°C (68°-77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol, cetyl alcohol, cetyl palmitate, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, stearyl alcohol.
| KAISER PERMANENTE
TERBINAFINE HYDROCHLORIDE
terbinafine hydrochloride cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Kaiser Foundation Hospitals (053052619) |
| Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | manufacture(0179-8715) | |
Revised: 11/2018
Document Id: 7b0aea92-ee73-8b64-e053-2991aa0aa6f1
Set id: 2a342421-fc4b-4c21-a394-5ddb9d4a96d5
Version: 2
Effective Time: 20181119
Kaiser Foundation Hospitals