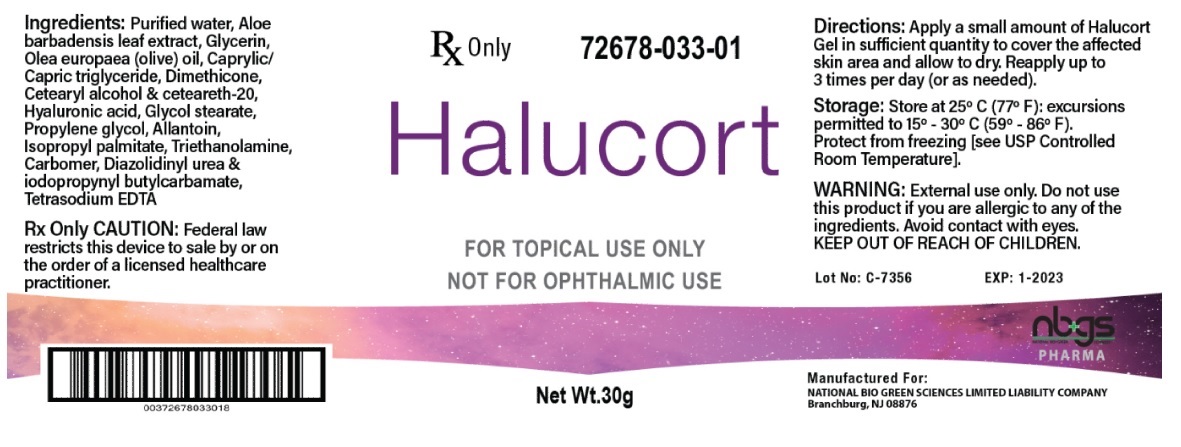
Label: HALUCORT GEL DRESSING WITH HYALURONIC ACID-
- NHRIC Code(s): 72678-033-01
- Packager: NATIONAL BIO GREEN SCIENCES LIMITED LIABILITY COMPANY
- Category: PRESCRIPTION MEDICAL DEVICE LABEL
Drug Label Information
Updated January 15, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
DESCRIPTION: Halucort is a soothing wound gel that is formulated for the dressing and management of superficial wounds, minor abrasions, pressure ulcers stage II - IV, 1st and 2nd degree burns, including sunburns, and radiation dermatitis. When applied properly to a wound hyaluronic acid adheres to tissue to prevent damage while supplying moisture to the cells. Medium molecular weight polysaccharides in aloe Vera moisturize and reduce inflammation. Proven therapeutic moisturizers, lipids, glycerin and allantoin, relieves dryness
-
INDICATIONS FOR USE
Under the supervision of a healthcare professional, Halucort Gel is indicated to manage and relieve the burning, itching and pain experienced with various types of dermatoses, including atopic dermatitis, allergic contact dermatitis and radiation dermatitis. Halucort Gel also helps to relieve dry, waxy skin by maintaining a moist wound and skin environment, which is beneficial to the healing process
- DESCRIPTION
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS AND OBSERVATIONS
- INSTRUCTIONS FOR USE
- INGREDIENTS
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
HALUCORT GEL DRESSING WITH HYALURONIC ACID
dressing, wound, occlusiveProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) NHRIC:72678-033 Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) OLIVE OIL (UNII: 6UYK2W1W1E) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIMETHICONE (UNII: 92RU3N3Y1O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) HYALURONIC ACID (UNII: S270N0TRQY) GLYCOL STEARATE (UNII: 0324G66D0E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALLANTOIN (UNII: 344S277G0Z) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) EDETATE SODIUM (UNII: MP1J8420LU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:72678-033-01 30 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device NAD 01/13/2020 Labeler - NATIONAL BIO GREEN SCIENCES LIMITED LIABILITY COMPANY (967054623)