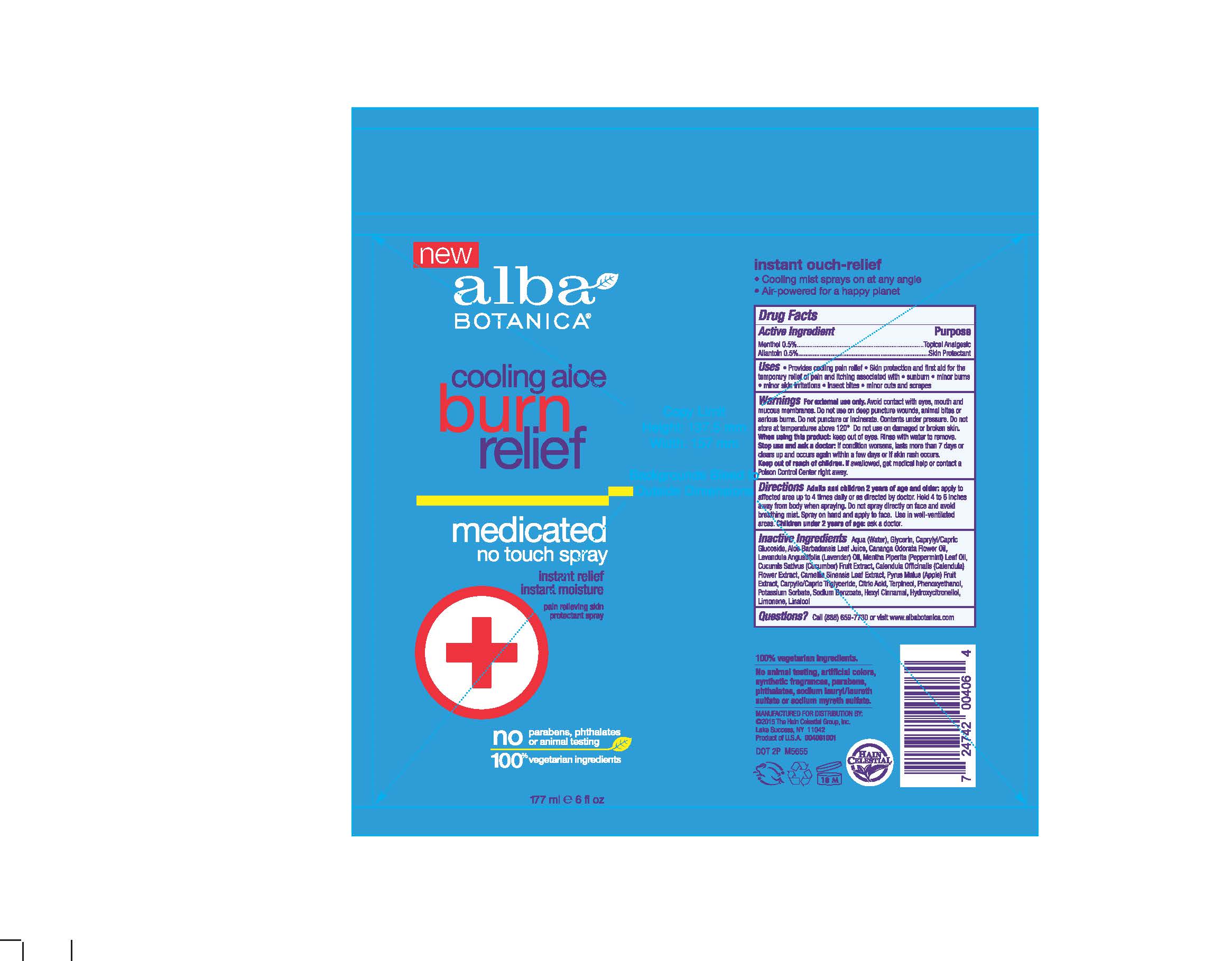
Label: AL0040600 ALBA COOLING ALOE BURN RELIEF- menthol, allantoin spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 61995-2406-6 - Packager: The Hain Celestial Group, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 5, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only. Avoid contact with eyes, mouth and mucous membranes. Do not use on deep puncture wounds, animal bites or serious burns. Do not puncture or incinerate. Contents under preassure .Do not store at temperature above 120°. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask doctor if condition worsens, lasts more than 7days or clears up and occurs again in within a few days or if skin rash occurs.
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Adults and children 2 years old and older: apply to affected area up to 4 times daily or as directed by doctor. Hold 4-6 inches away from body when spraying. Do not spray directly on face and avoid breathing mist. Sprayon hand and apply to face.Use in well-ventiled area. Children under 2 years of age : ask a doctor.
-
INACTIVE INGREDIENT
Aquq (Water),Glycerin, Caprylyl/Capric Glucoside, Aloe Barbadensis Leaf Juice, Cananga Odarata Flower Oil, Mentha Piperita (Peppermint) Oil, Cucumis Sativus (Cucumber) Fruit Extract, Calendula Officinalis Flower Extract, Camellia Sinensis Leaf Extract, Pyrus Malus (Apple) Fruit Extract, Caprylic/Capric Trigluceride, Citric Acid, Terpineol, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate, Hexyl Cinnamal, Hydroxycitronellol, Limonene, Linalool.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AL0040600 ALBA COOLING ALOE BURN RELIEF
menthol, allantoin sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61995-2406 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.5 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CANANGA OIL (UNII: 8YOY78GNNX) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) APPLE (UNII: B423VGH5S9) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) LAVENDER OIL (UNII: ZBP1YXW0H8) CUCUMBER (UNII: YY7C30VXJT) TERPINEOL (UNII: R53Q4ZWC99) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYDROXYCITRONELLOL (UNII: R0B4U2I48W) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) PEPPERMINT OIL (UNII: AV092KU4JH) SODIUM BENZOATE (UNII: OJ245FE5EU) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GREEN TEA LEAF (UNII: W2ZU1RY8B0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61995-2406-6 177 g in 1 CAN; Type 0: Not a Combination Product 01/13/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 01/13/2016 Labeler - The Hain Celestial Group, Inc (117115556) Registrant - The Hain Celestial Group, Inc. (014334364) Establishment Name Address ID/FEI Business Operations The Hain Celestial Group, Inc 081512382 manufacture(61995-2406)