CHAVITA 4- aur met, cetraria, ferrum met, zinc val, crataegus, eucalyptus globulus, thymus gland liquid
RUBIMED AG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Chavita 4
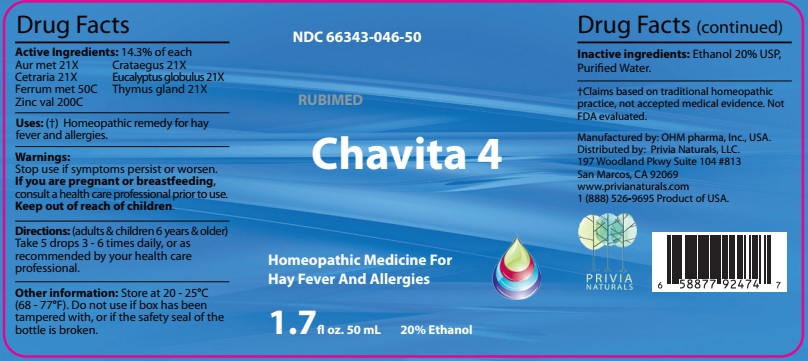
Drug Facts
Active Ingredients: 14.3% of each
Aur met 21X Crataegus 21X
Cetraria 21X Eucalyptus globulus 21X
Ferrum met 50C Thymus gland 21X
Zinc val 200C
† Claims based on traditional homeopathic
practice, not accepted medical evidence. Not
FDA evaluated.
Warnings:Stop use if symptoms persist or worsen.
If you are pregnant or breastfeeding,
consult a health care professional prior to use.
Keep out of reach of children.
Directions: (adults & children 6 years & older)
Take 5 drops 3 - 6 times daily, or as
recommended by your health care professional.
Other information: Store at 20 - 25°C
(68 - 77ºF). Do not use if box has been
tampered with, or is the safety seal of the
bottle is broken.
| CHAVITA 4
aur met, cetraria, ferrum met, zinc val, crataegus, eucalyptus globulus, thymus gland liquid |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - RUBIMED AG (480582035) |