SCRUB CARE EXIDINE-2 CHG- chlorhexidine gluconate solution
Xttrium Laboratories, Inc.
----------
Drug Facts 2% 30 oz
Uses
- surgical hand scrub: significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
- healthcare personnel handwash: helps reduce bacteria that potentially can cause disease
Warnings
For external use only
Alergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums
- if solution should contact these areas, rinse out promptly and thoroughly with water
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
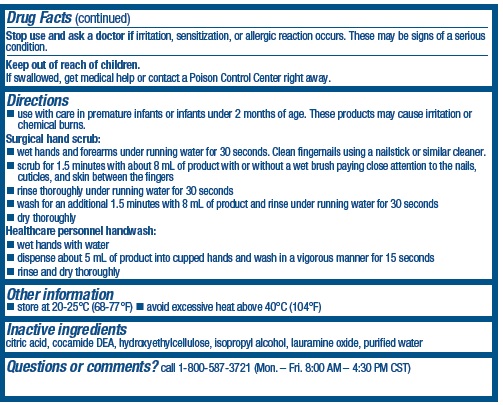
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms under running water for 30 seconds. Clean fingernails using a nailstick or similar cleaner.
- scrub for 1.5 minutes with about 8 ml of product with or without a wet brush paying close attention to the nails, cuticles, and skin between the fingers
- rinse thoroughly under running water for 30 seconds
- wash an additional 1.5 minutes with 8 ml of product and rinse under running water for 30 seconds
- dry thoroughly
Healthcare personnel handwash:
- wet hands with water
- dispense about 5 ml of product into cupped hands and wash in a vigorous manner for 15 seconds
- rinse and dry thouroughly
Inactive ingredients
citric acid, cocamide DEA, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, purified water
Package Label
Cat. 4CHG2 NDC 0116-4242-30
Scrub Care
Exidine -2 CHG 2%
Chlorhexidine Gluconate 2% Solution
Antiseptic
Contains: 2% Chlorhexidine Gluconate
For External Use Only
Distributed By
CareFusion, Inc.
Vernon Hills, IL 60061 USA
51-10102
2CF30BTLLBLB
Net Contents: 30 fl oz (887 ml)
CareFusion
To Install
1. Twist to remove plug from back of dispenser spout.
2. Seat dispenser into bracket, taking care to insert the open air inlet into proper receptacle.
3. Twist to remove plug from end of spout.
To remove
1. Hold bracket against the wall and pull the empty dispenser straight froward.
2. Discard entire bottle and spout.


| SCRUB CARE
EXIDINE-2 CHG
chlorhexidine gluconate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Xttrium Laboratories, Inc. (007470579) |
| Registrant - Xttrium Laboratories, Inc. (007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Xttirum Laboratories, Inc. | 007470579 | manufacture(0116-4242) | |