Label: CHILDRENS LORATADINE- loratadine solution
- NDC Code(s): 62011-0305-1
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 26, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL teaspoonful)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
-
Directions
- use only with enclosed dosing cup
adults and children 6 years and over 2 teaspoonfuls (tsp) daily; do not take more than 2 teaspoonfuls (tsp) in 24 hours children 2 to under 6 years of age 1 teaspoonful (tsp) daily; do not take more than 1 teaspoonful (tsp) in 24 hours children under 2 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
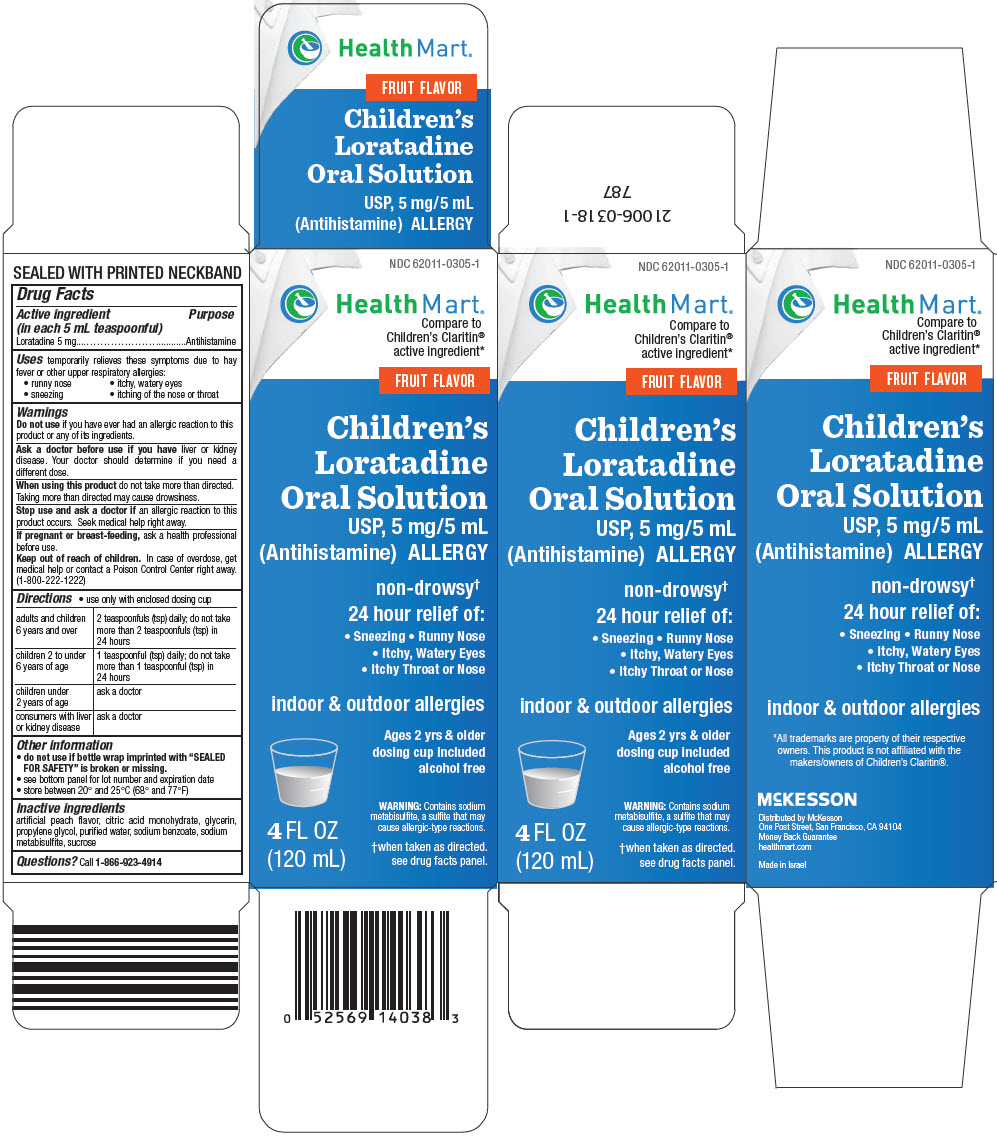
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Carton
NDC 62011-0305-1
Health Mart®
Compare to
Children's Claritin®
active ingredient*FRUIT FLAVOR
Children's
Loratadine
Oral Solution
USP, 5 mg/5 mL
(Antihistamine) ALLERGYnon-drowsy†
24 hour relief of:- Sneezing • Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
indoor & outdoor allergies
Ages 2 yrs & older
dosing cup included
alcohol freeWARNING: Contains sodium
metabisulfite, a sulfite that may
cause allergic-type reactions.†when taken as directed.
see drug facts panel.4 FL OZ
(120 mL)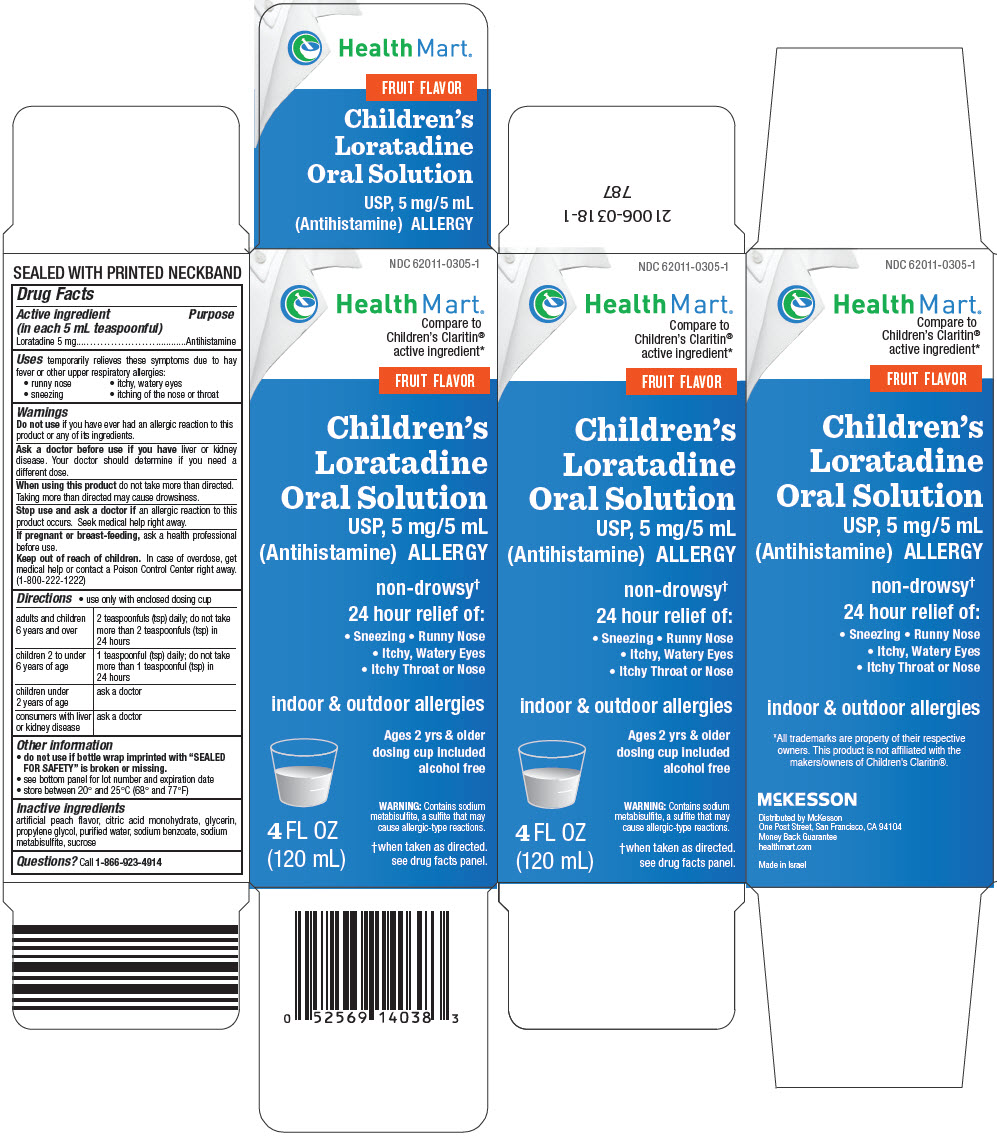
-
INGREDIENTS AND APPEARANCE
CHILDRENS LORATADINE
loratadine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62011-0305 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Loratadine (UNII: 7AJO3BO7QN) (Loratadine - UNII:7AJO3BO7QN) Loratadine 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength citric acid monohydrate (UNII: 2968PHW8QP) glycerin (UNII: PDC6A3C0OX) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) sodium benzoate (UNII: OJ245FE5EU) sodium metabisulfite (UNII: 4VON5FNS3C) sucrose (UNII: C151H8M554) Product Characteristics Color YELLOW (colorless to slightly yellow) Score Shape Size Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62011-0305-1 1 in 1 CARTON 11/15/2016 1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076805 08/20/2004 Labeler - Strategic Sourcing Services LLC (116956644)