DELSYM- dextromethorphan suspension, extended release
RB Health (US) LLC
----------
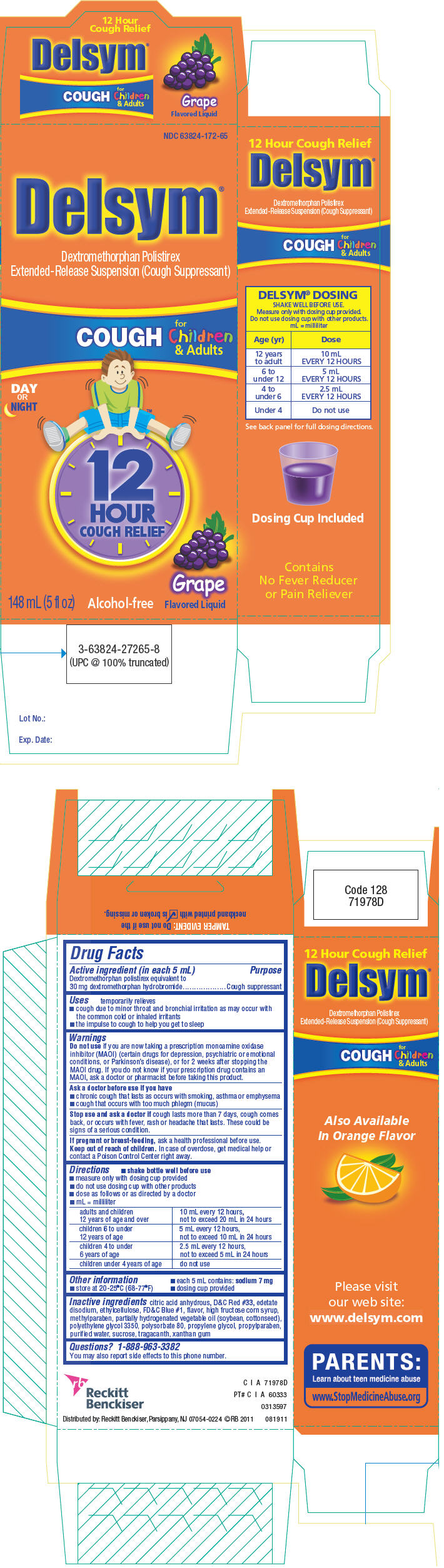
Delsym
12 Hour Cough Relief
Grape
for Children and Adults
Active ingredient (in each 5 mL)
Dextromethorphan polistirex equivalent to 30 mg dextromethorphan hydrobromide
Uses
temporarily relieves
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the impulse to cough to help you get to sleep
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- chronic cough that lasts as occurs with smoking, asthma or emphysema
- cough that occurs with too much phlegm (mucus)
Directions
- shake bottle well before use
- measure only with dosing cup provided. Do not use dosing cup with other products.
- dose as follows or as directed by a doctor
- mL = milliliter
| adults and children 12 years of age and over | 10 mL every 12 hours,
not to exceed 20 mL in 24 hours |
| children 6 to under 12 years of age | 5 mL every 12 hours,
not to exceed 10 mL in 24 hours |
| children 4 to under 6 years of age | 2.5 mL every 12 hours,
not to exceed 5 mL in 24 hours |
| children under 4 years of age | do not use |
| DELSYM
dextromethorphan suspension, extended release |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |
Revised: 5/2023
Document Id: fbbf4fd1-a810-fc54-e053-6294a90af912
Set id: 28002d7e-335c-43d0-ac88-7bca1efb7ddf
Version: 5
Effective Time: 20230515
RB Health (US) LLC