MARY KAY TIMEWISE BODY HAND AND DECOLETTE CREAM SPF 15- avobenzone, octisalate, octocrylene, oxybenzone cream
Mary Kay Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
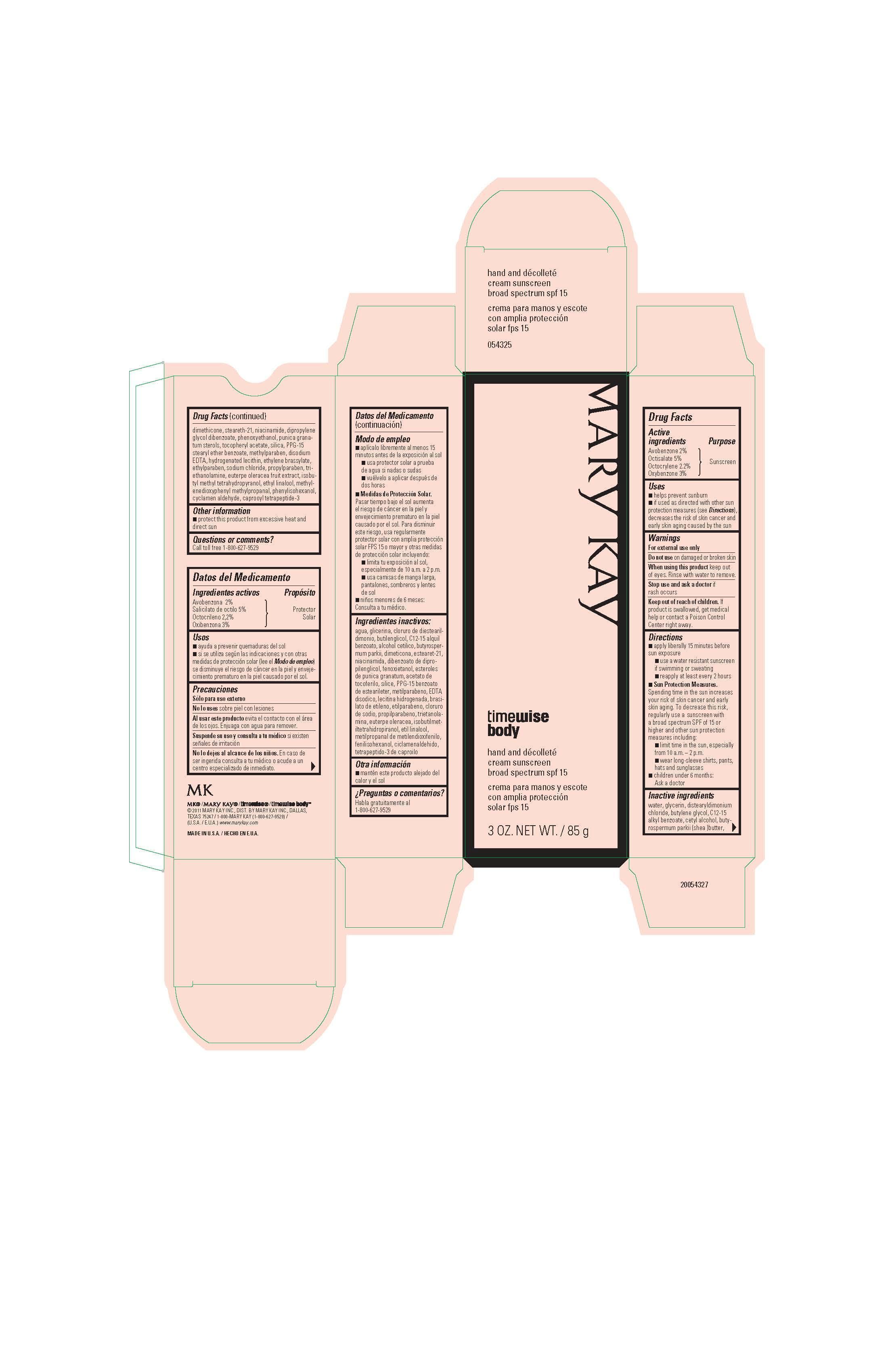
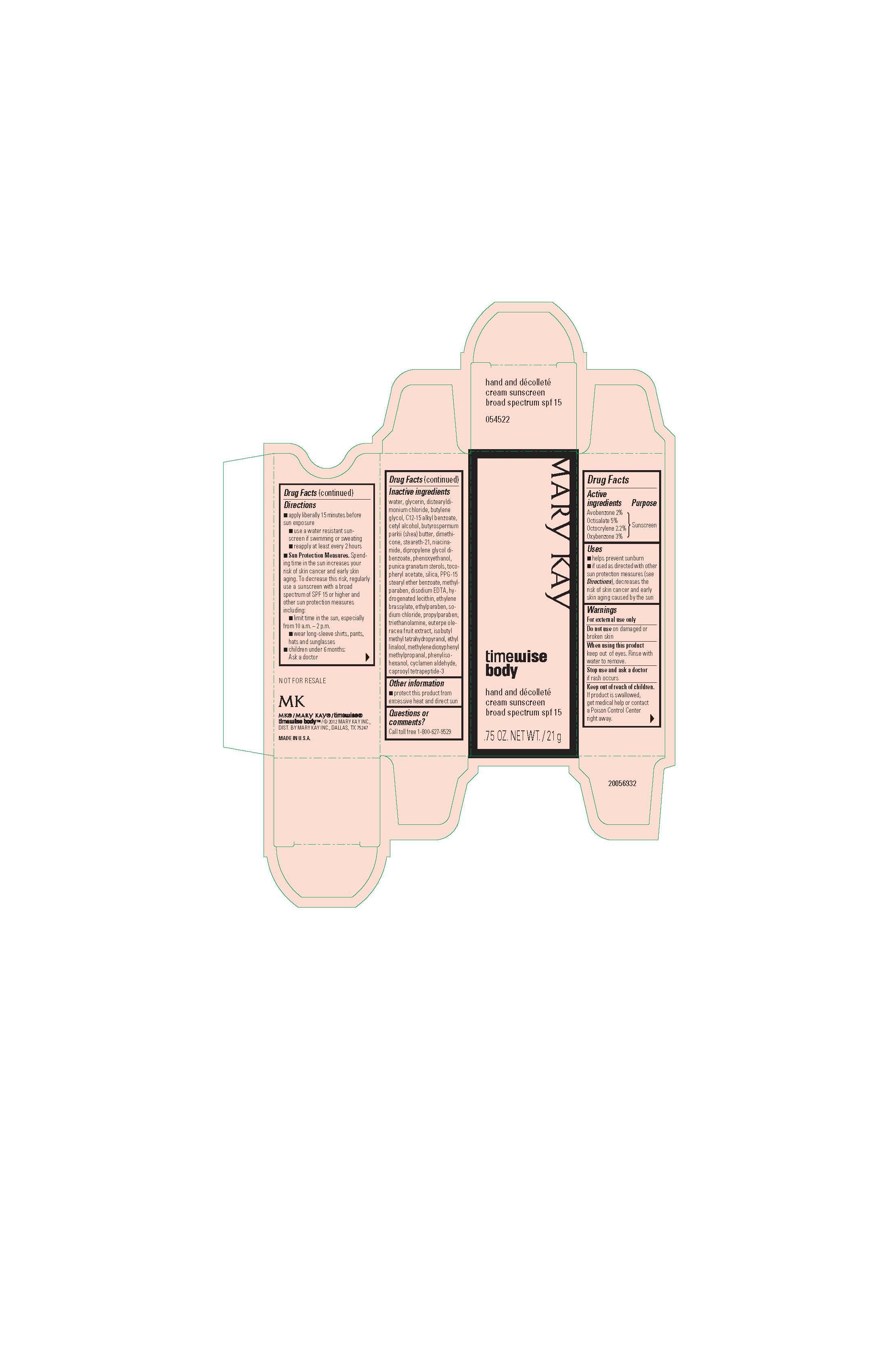
Mary Kay TimeWise Body Hand and Decolette Cream SPF 15
Drug Facts
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply liberally 15 minutes before sun exposure
- use a water-resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive ingredients
water, glycerin, distearyldimonium chloride, butylene glycol, C12-15 alkyl
benzoate, cetyl alcohol, butyrospermum parkii (shea) butter, dimethicone,
steareth-21, niacinamide, dipropylene glycol dibenzoate, phenoxyethanol,
punica granatum sterols, tocopheryl acetate, silica, PPG-15 stearyl ether benzoate,
methylparaben, disodium EDTA, hydrogenated lecithin, ethylene brassylate, ethylparaben,
sodium chloride, propylparaben, triethanolamine, euterpe oleracea fruit extract,
isobutyl methyl tetrahydropyranol, ethyl linalool, methylenedioxyphenyl methylpropanal,
phenylisohexanol, cyclamen aldehyde, caprooyl tetrapeptide-3
| MARY KAY TIMEWISE BODY HAND AND DECOLETTE CREAM SPF 15
avobenzone, octisalate, octocrylene, oxybenzone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Mary Kay Inc. (049994452) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Mary Kay Inc. | 103978839 | manufacture(51531-4325) | |