VALUMEDS ASPIRIN FREE HEADACHE RELIEF PM- acetaminophen and diphenhydramine citrate tablet, coated
SPIRIT PHARMACEUTICALS LLC
----------
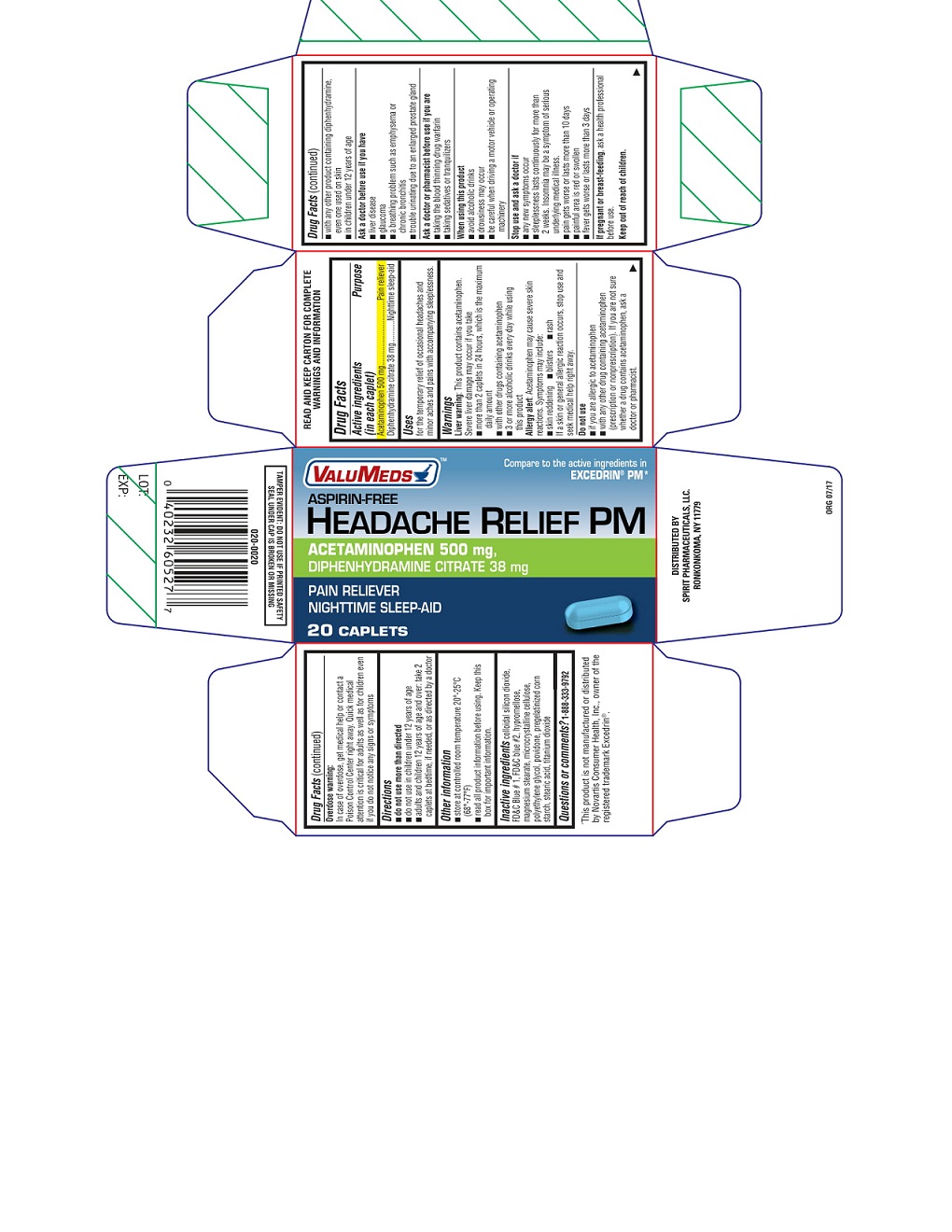
VALUMEDS ASPIRIN FREE HEADACHE RELIEF PM
Uses
for the temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness.
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 2 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Do not use
- if you are allergic to acetaminophen
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
Ask a doctor before use if you have
- liver disease
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- avoid alcoholic drinks
- drowsiness may occur
- be careful when driving a motor vehicle or operating machinery
Directions
- do not use more than directed
- do not use in children under 12 years of age
- adults and children 12 years of age and over: take 2 caplets at bedtime, if needed, or as directed by a doctor
Other information
- store at controlled room temperature 20°-25°C (68°-77°F)
- read all product information before using. Keep this box for important information.
| VALUMEDS ASPIRIN FREE HEADACHE RELIEF PM
acetaminophen and diphenhydramine citrate tablet, coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - SPIRIT PHARMACEUTICALS LLC (179621011) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ELYSIUM PHARMACEUTICALS LIMITED | 915664486 | manufacture(68210-2000) | |
Revised: 12/2023
Document Id: 0bf3d436-7c13-f6bc-e063-6294a90a0bc2
Set id: 27d83e0f-344a-4527-b86f-a8c7db33a585
Version: 6
Effective Time: 20231207
SPIRIT PHARMACEUTICALS LLC