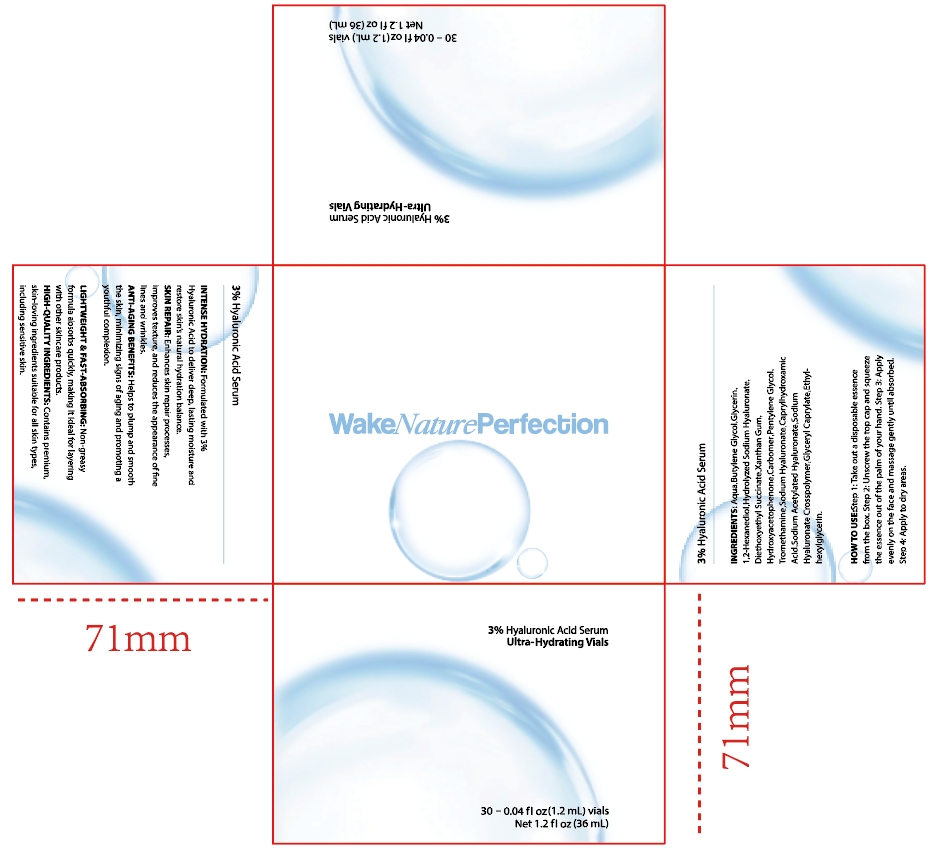
Label: 3% HYALURONIC ACID SERUM liquid
- NDC Code(s): 84732-095-01
- Packager: Dongguan Haiyi Technology Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Inactive ingredient
-
Purpose section
- INTENSE HYDRATION: Formulated with 3%Hyaluronic Acid to deliver deep, lasting moisture andrestore skin's natural hydration balance.
- SKIN REPAIR: Enhances skin repair processes,improves texture, and reduces the appearance of fnelines and wrinkles.
- ANTI-AGING BENEFITS: Helps to plump and smooththe skin, minimizing signs ofaging and promoting ayouthful complexion.
- LIGHTWEIGHT&FAST-ABSORBING: Non-greasyformula absorbs quickly, making it ideal for layeringwith other skincare products.HIGH-QUALITYINGREDIENTS: Contains premium,skin-loving ingredients suitable for all skin types,including sensitive skin.
- Warning
- stop use
- not use
- OUT OF CHILDREN
- HOW TO USE
- Dosage
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
3% HYALURONIC ACID SERUM
3% hyaluronic acid serum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84732-095 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) (BUTYLENE GLYCOL - UNII:3XUS85K0RA) BUTYLENE GLYCOL 1 mg in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) DIETHOXYETHYL SUCCINATE (UNII: R1B8ZV94R9) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYALURONATE (UNII: YSE9PPT4TH) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84732-095-01 199 g in 1 BOX; Type 0: Not a Combination Product 11/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/25/2024 Labeler - Dongguan Haiyi Technology Co.,Ltd. (722030807) Establishment Name Address ID/FEI Business Operations Dongguan Haiyi Technology Co.,Ltd. 722030807 manufacture(84732-095)