VANAHIST PD- triprolidine hydrochloride liquid
GM Pharmaceuticals, INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Vanahist PD
Vanahist PD
Tamper evident by foil seal under cap.Do not use if foil seal is broken or missing.
Distributed by: GM Pharmaceuticals, Inc.
Arlington, TX 76015 R102816
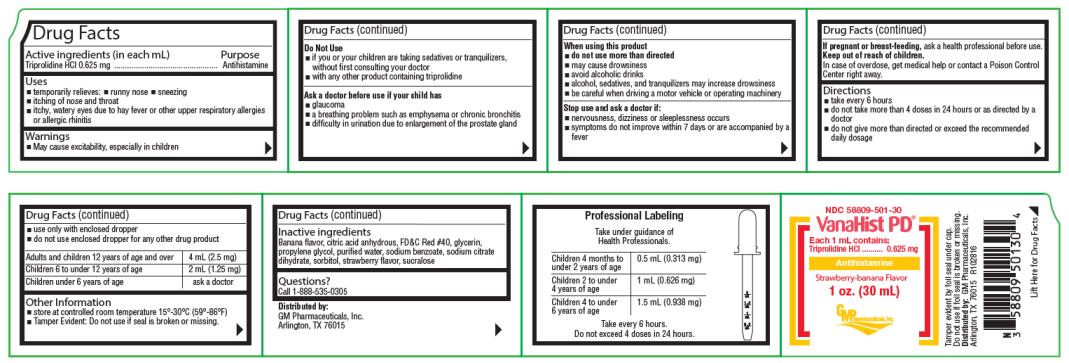
Uses
- temporarily relieves:
- runny nose
- sneezing
- itching of nose and throat
- itchy, watery eyes due to hay fever or other upper respiratory allergies or allergic rhinitis
Warnings
- May cause excitability, especially in children
Do Not Use
- if you or your children are taking sedatives or tranquilizers, without first consulting your doctor
- with any other product containing triprolidine
Ask a doctor before use if your child has
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
When using this product
- do not use more than directed
- may cause drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Directions
- take every 6 hours
- do not take more than 4 doses in 24 hours or as directed by a doctor
- do not give more than directed or exceed the recommended daily dosage
- use only with enclosed dropper
- do not use enclosed dropper for any other drug product
| Adults and children 12 years of age and over | 4 mL (2.5 mg) |
| Children 6 to under 12 years of age | 2 mL (1.25 mg) |
| Children under 6 years of age | ask a doctor |
Professional Labeling
Take under guidance of Health Professionals.
| Children 4 months to under 2 years of age | 0.5 mL (0.313 mg) |
| Children 2 to under 4 years of age | 1 mL (0.626 mg) |
| Children 4 to under 6 years of age | 1.5 mL (0.938 mg) |
Take every 6 hours.
Do not exceed 4 doses in 24 hours.
| VANAHIST PD
triprolidine hydrochloride liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - GM Pharmaceuticals, INC (793000860) |
Revised: 11/2019
Document Id: 1e3b6bda-7695-47dc-bf82-3695fb6f49ae
Set id: 256f6149-8a53-4dee-b25a-43197804946a
Version: 3
Effective Time: 20191105
GM Pharmaceuticals, INC