COCAINE HYDROCHLORIDE- cocaine hydrochloride solution
Lannett Company, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
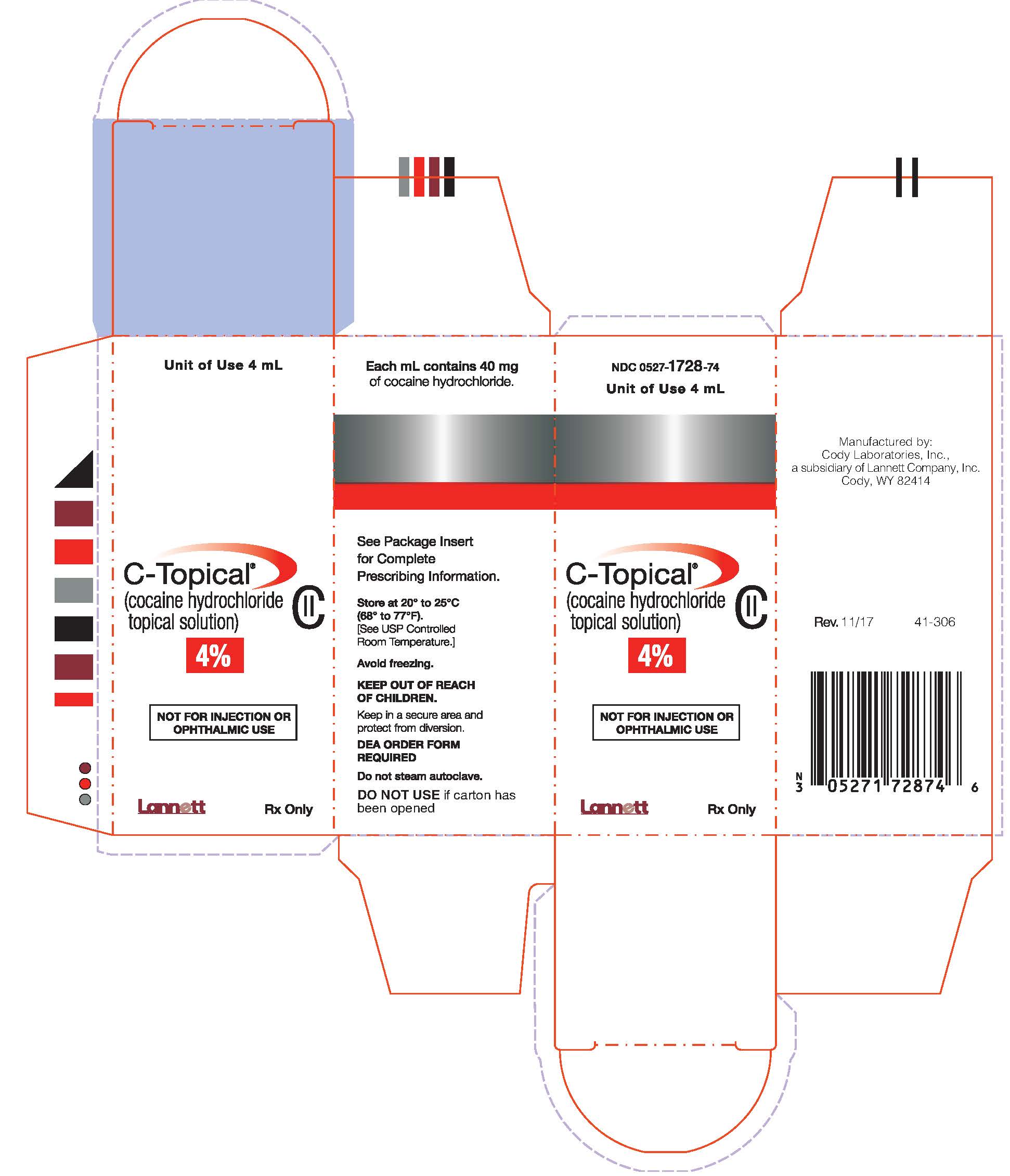
COCAINE HYDROCHLORIDE Topical Solution CII
Rx only
DESCRIPTION
Each mL contains:
Cocaine hydrochloride 40 mg or 100 mg as aqueous solution.
The topical solution contains the following inactive ingredients: citric acid, D&C Yellow No. 10, FD&C Green No. 3, sodium benzoate, and water.
NOTE (for Glass Bottle): External surface of unopened bottle may be sterilized by ethylene oxide only. Do not steam autoclave.
Cocaine hydrochloride USP is a crystalline, granular, or powder substance having a saline, slightly bitter taste that numbs tongue and lips. Cocaine hydrochloride is a local anesthetic.
CLINICAL PHARMACOLOGY
Cocaine blocks the initiation or conduction of the nerve impulse following local application, thereby effecting local anesthetic action.
Cocaine is absorbed from all sites of application, including mucous membranes and the gastrointestinal mucosa. Cocaine is degraded by plasma esterases, with the half-life in the plasma being approximately one hour.
INDICATIONS AND USAGE
Cocaine hydrochloride topical solution is indicated for the introduction of local (topical) anesthesia of accessible mucous membranes of the oral, laryngeal and nasal cavities.
CONTRAINDICATIONS
Cocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to the drug or to the components of the topical solution.
WARNINGS
RESUSCITATIVE EQUIPMENT AND DRUGS SHOULD BE IMMEDIATELY AVAILABLE WHEN ANY LOCAL ANESTHETIC IS USED.
Carcinogenesis, Mutagenesis
Long-term studies to determine the carcinogenic and mutagenic potential of cocaine are not available.
Pregnancy
Teratogenic Effects-Pregnancy Category C:
Animal reproduction studies have not been conducted with cocaine. It is also not known whether cocaine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Cocaine should be given to a pregnant woman only if needed.
PRECAUTIONS
The safety and effectiveness of cocaine hydrochloride topical solution depends on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be consulted for specific techniques and precautions for various anesthetic procedures.
The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age and physical status.
Cocaine hydrochloride topical solution should be used with caution in patients with severely traumatized mucosa and sepsis in the region of the proposed application. Use with caution in persons with known drug sensitivities.
ADVERSE REACTIONS
Adverse reactions may be due to high plasma levels as a result of excessive and rapid absorption of the drug. Reactions are systemic in nature and involve the central nervous system and/or the cardiovascular system. A small number of reactions may result from hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient.
CNS reactions are excitatory and/or depressant, and may be characterized by nervousness, restlessness and excitement. Tremors and eventually clonic-tonic convulsions may result. Emesis may occur. Central stimulation is followed by depression, with death resulting from respiratory failure.
Small doses of cocaine slow the heart rate, but after moderate doses, the rate is increased due to central sympathetic stimulation.
Cocaine is pyrogenic, augmenting heat production in stimulating muscular activity and causing vasoconstriction which decreases heat loss. Cocaine is known to interfere with the uptake of norepinephrine by adrenergic nerve terminals, producing sensitization to catecholamines, causing vasoconstriction and mydriasis.
Cocaine causes sloughing of the corneal epithelium, causing clouding, pitting, and occasionally ulceration of the cornea. The drug is not meant for ophthalmic use.
OVERDOSAGE
The fatal dose of cocaine has been approximated at 1.2 g., although severe toxic effects have been reported from doses as low as 20 mg.
Symptoms - The symptoms of cocaine poisoning are referable to the CNS, namely the patient becomes excited, restless, garrulous, anxious and confused. Enhanced reflexes, headache, rapid pulse, irregular respiration, chills, rise in body temperature, mydriasis, exophthalmos, nausea, vomiting and abdominal pain are noticed. In severe overdoses, delirium, Cheyne-Stokes respiration, convulsions, unconsciousness, and death from respiratory arrest result. Acute poisoning by cocaine is rapid in developing.
Treatment - The specific treatment of acute cocaine poisoning is the intravenous administration of a short-acting barbiturate or diazepam. Artificial respiration may be necessary. It is important to limit absorption of the drug. If entrance of the drug into circulation can be checked, and respiratory exchange maintained, the prognosis is favorable since cocaine is eliminated fairly rapidly.
DOSAGE AND ADMINISTRATION
The dosage varies and depends upon the area to be anesthetized, vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dosage needed to provide effective anesthesia should be administered. Dosages should be reduced for children and for elderly and debilitated patients. Cocaine hydrochloride topical solution can be administered by means of cotton applicators or packs, instilled into a cavity, or as a spray.
HOW SUPPLIED
4% Cocaine Hydrochloride Topical Solution, clear, blue-green solution.
NDC 0527-1728-74: Unit-of-Use Glass Bottle filled to contain 4 mL, one 4 mL bottle per carton.
NDC 0527-1728-73: Multi-Dose Bottle of 10 mL.
10% Cocaine Hydrochloride Topical Solution, clear, blue-green solution.
NDC 0527-1729-74: Unit-of-Use Glass Bottle filled to contain 4 mL, one 4 mL bottle per carton.
NDC 0527-1729-73: Multi-Dose Bottle of 10 mL.
DEA Order Form Required.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
Avoid freezing.
Keep out of reach of children.
Manufactured by:
Cody Laboratories, Inc.
a subsidiary of Lannett Company, Inc.
Cody, WY 82414
Revision 04/19 11-304
| COCAINE HYDROCHLORIDE
cocaine hydrochloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| COCAINE HYDROCHLORIDE
cocaine hydrochloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Lannett Company, Inc. (002277481) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cody Laboratories, Inc. | 028153216 | ANALYSIS(0527-1728, 0527-1729) , LABEL(0527-1728, 0527-1729) , MANUFACTURE(0527-1728, 0527-1729) , PACK(0527-1728, 0527-1729) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lannett Company, Inc. | 829757603 | ANALYSIS(0527-1728, 0527-1729) | |