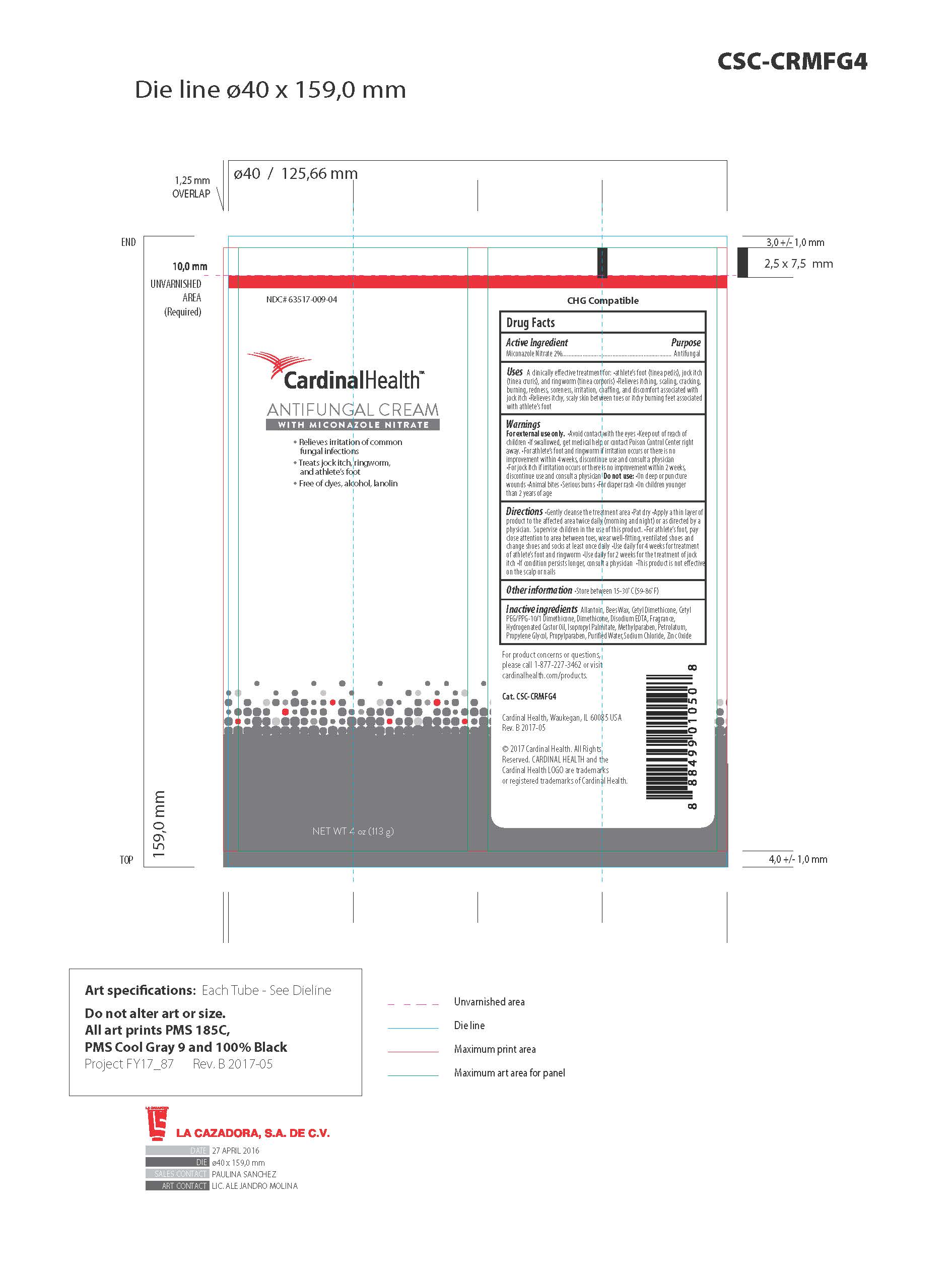
ANTIFUNGAL CREAM- 2% miconazole nitrate cream cream
Cardinal Health, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Antifungal Cream
Warnings
- For external use only
- Avoid contact with eyes
- Keep out of reach of children
- If swallowed, get medical help or contact Poison Control Center right away
- For athlete's foot and ringworm, if irritation occurs or there is no improvement within 4 weeks, discontinue use and consult a physician
- For jock itch, if irritation occurs or there is no improvement within 2 weeks, discontinue use and consult a physician
- Do not use on deep or puncture wounds, animal bites, serious burns, for diaper rash, on children younger tha 2 years of age.
Uses
A clinically effective treatment for:
- most athlete's foot (tinea pedis)
- jock itch (tinea cruris)
- ringworm (tinea corporis)
Relieves itching, scaling, cracking, burning, redness, soreness, irritation, discomfort and chafing associated with jock itch
Uses
A clinically effective treatment for:
most athlete's foot (tinea pedis)jock itch (tinea cruris)ringworm (tinea corporis)
Relieves itching, scaling, cracking, burning, redness, soreness, irritation, discomfort and chafing associated with jock itch
Directions
- Glently cleanse the treatment area
- Pat dry
- Apply a thin layer of product to the affected area twice daily (morning and night) or as directed by a physician
- For athlete's foot, pay close attention to area between toes, wear well-fitting ventilated shoes, and change shoes and socks at least once daily
- Use daily for 4 weeks for treatment of athlete's foot and ringworm
- Use daily for 2 weeks for treamnet of jock itch
- If condition persists longer, consult a physician
- This product is not effective on the scalp or nails
Directions
Glently cleanse the treatment areaPat dryApply a thin layer of product to the affected area twice daily (morning and night) or as directed by a physicianFor athlete's foot, pay close attention to area between toes, wear well-fitting ventilated shoes, and change shoes and socks at least once dailyUse daily for 4 weeks for treatment of athlete's foot and ringwormUse daily for 2 weeks for treamnet of jock itchIf condition persists longer, consult a physicianThis product is not effective on the scalp or nails
| ANTIFUNGAL CREAM
2% miconazole nitrate cream cream |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Cardinal Health, Inc. (961027315) |