DIHYDROERGOTAMINE MESYLATE- dihydroergotamine mesylate injection
Hikma Farmaceutica
----------
DIHYDROERGOTAMINE MESYLATE INJECTION, USP
Rx only
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PRINCIPAL DISPLAY PANEL
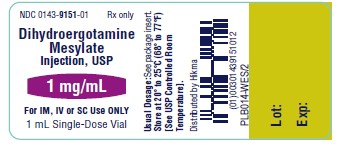
NDC 0143-9151-01 Rx only
Dihydroergotamine
Mesylate Injection, USP
1 mg/mL
For IV, IM or SC Use ONLY
1 mL Single-Dose Vial
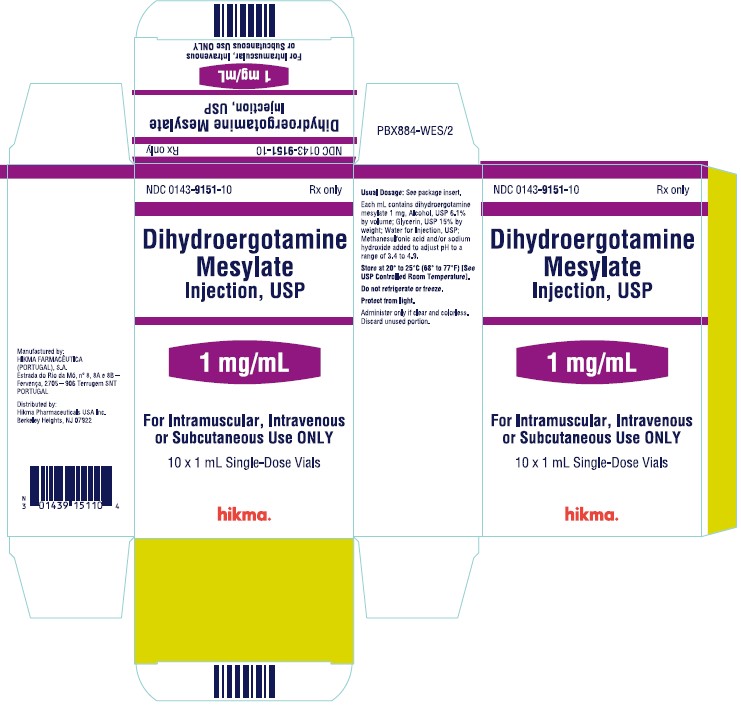
NDC 0143-9151-10 Rx only
Dihydroergotamine
Mesylate Injection, USP
1 mg/mL
For Intramuscular, Intravenous or Subcutaneous Use ONLY
10 x 1 mL Single-Dose Vials
| DIHYDROERGOTAMINE MESYLATE
dihydroergotamine mesylate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hikma Farmaceutica (452742943) |
| Registrant - Hikma Pharmaceuticals USA Inc. (946499746) |
Revised: 2/2024
Document Id: 1f3632e0-09d7-4c7f-9066-0652402362bc
Set id: 2345c909-d0d5-4403-9f6c-4f425366a9ab
Version: 3
Effective Time: 20240207
Hikma Farmaceutica