PAIN RELIEF BALM- menthol ointment
BARKER WELLNESS LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BARKER WELLNESS - PAIN RELIEF BALM - 3.5% (82383-104) DELIST
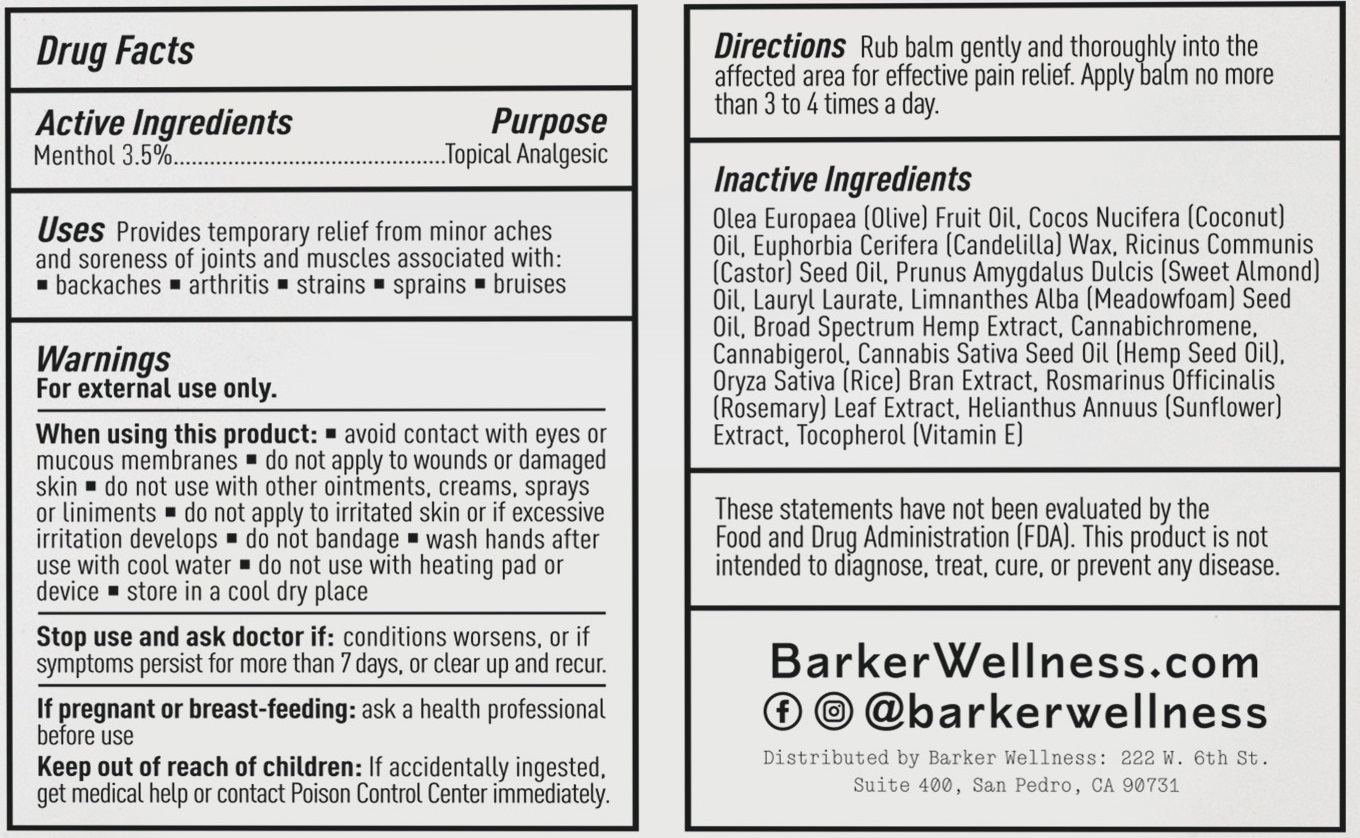
USES
Provides temporary relief from minor aches and soreness of joints and muscles associated with:
- backaches
- arthritis
- strains
- sprains
- bruises
WARNINGS
For external use only.
When using this product
- Avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged skin
- do not use with other ointments, creams, sprays or liniments
- do not apply to irritated skin or if excessive irritation develops
- do not bandage
- wash hands after use with cool water
- do not use with heating pad or device
- store in a cool dry place
Stop use and ask doctor if: condition worsens, or if symptoms persist for more than 7 days, or clear up and recur.
If pregnant or breast feeding: ask a health professional befgore use.
Keep out of reach of children. If accidentally ingested, get medical help or contact Poison Control Center immediatley
DIRECTIONS
Rub balm gently and thoroughly into the affected area for effective pain relief. Apply cream no more than 3 to 4 times a day.
INACTIVE INGREDIENTS
Olea Europaea (Olive) Fruit Oil, Cocos Nucifera (Coconut) Oil, Euphorbia Cerifera (Candelilla) Wax. Ricinus Communis (Castor) Seed Oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Lauryl Laurate, Limnanthes Alba (Meadowfoam) Seed Oil, Broad Spectrum Hemp Extract, Cannabichromene. Cannabigerol, Cannabis Saliva Seed Oil (Hemp Seed Dill. Oryza Sativa (Rice) Bran Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Helianthus Annuus (Sunflower) Extract, Tocopherol (Vitamin E)
| PAIN RELIEF BALM
menthol ointment |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - BARKER WELLNESS LLC (108169543) |