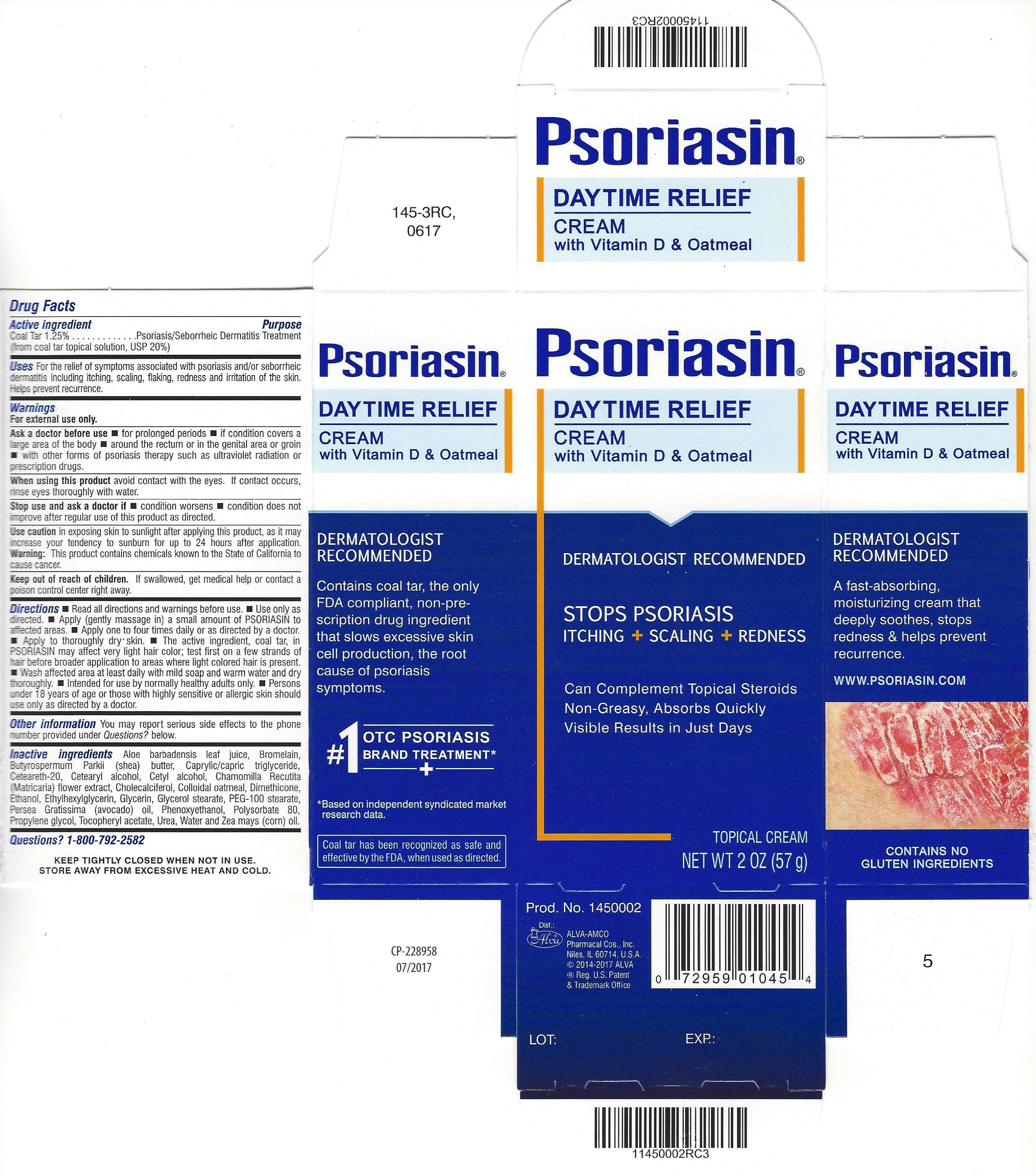
PSORIASIN DAYTIME RELIEF- coal tar cream
Alva-Amco Pharmacal Companies, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
For the relief of symptoms associated with psoriasis and/or seborrheic dermatitis including itching, scaling, flaking, redness and irritation of the skin. Helps prevent recurrence.
Ask a doctor before use
- for prolonged periods
- if condition covers a large area of the body
- around the rectum or in the genital area or groin
- with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs
When using this product avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- condition worsens
- condition does not improve after regular use of this product as directed.
Use caution in exposing skin to sunlight after applying this product, as it may increase your tendency to sunburn for up to 24 hours after application.
Warning: This product contains chemicals known to the State of California to cause cancer.
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Directions
- Read all package directions and warnings before use.
- Use only as directed.
- Apply (gently massage in) a small amount of Psoriasin to affected areas.
- Apply one to four times daily or as directed by a doctor.
- Apply to thoroughly dry skin.
- The active ingredient, coal tar, in Psoriasin, may affect very light hair color; test first on a few strands of hair before broader application to areas where light-colored hair is present.
- Wash affected area at least daily with mild soap and warm ater and dry thoroughly.
- Intended for use by normally healthy adults only.
- Persons under 18 years of age or those with highly sensitive or allergic skin should use only as directed by a doctor.
Other information
You may report serious side effectes to the phone number provided under Questions? below.
Inactive ingredients
Aloe barbadensis leaf juice, Bromelain, Butyrospermum Parkii (shea) butter, Caprylic/capric triglyceride, Ceteareth-20, Cetearyl alcohol, Cetyl alcohol, Chamomilla Recutita (Matricaria) flower extract, Cholecalciferol, Colloidal oatmeal, Dimethicone, Ethanol, Ethylhexylglycerin, Glycerin, Glycerol stearate, PEG-100 stearate, Persea Gratissima (avocado) oil, Phenoxyethanol, Polysorbate 80, Propylene glycol, Tocopheryl acetate, Urea, Water and Zea mays (corn) oil.
| PSORIASIN DAYTIME RELIEF
coal tar cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856) |