ARRID DRY INVIGORATE- aluminum chlorohydrate aerosol, spray
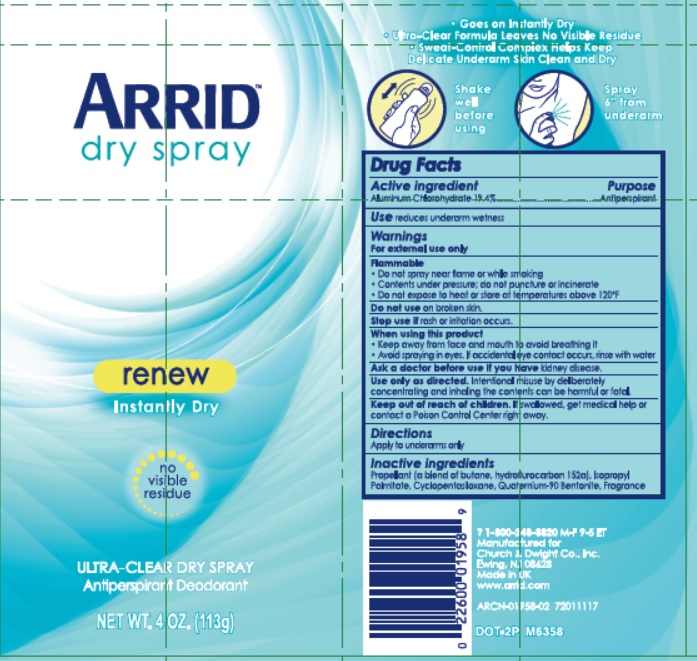
ARRID DRY RENEW- aluminum chlorohydrate aerosol, spray
Fillcare Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Arrid Dry Spray
Warnings
For external use only
Flammable
- Do not spray near flame or while smoking
- Contents under pressure do not puncture or incinerate
- Do not expose to heat or store at temperatures above 120F
Do not use on broken skin.
Stop use if rash or irritation occurs.
When using this product
- Keep away from face and mouth to avoid breathing it
- Avoid spraying in eyes. If accidental eye contact occurs, rinse with water.
Ask a doctor before use if you have kidney disease.
Use only as directed. Intentional misuse by deliberately concentrating and inhaling the contents can be harmful or fatal.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
Propellant (a blend of butane, hydrofluorocarbon 152a), Isopropyl Palmitate, Cyclopentasiloxane, Quaternium-90 Bentonite, Fragrance
| ARRID DRY
INVIGORATE
aluminum chlorohydrate aerosol, spray |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ARRID DRY
RENEW
aluminum chlorohydrate aerosol, spray |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Fillcare Limited (227632171) |
| Registrant - Church & Dwight Co., Inc. (001211952) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fillcare Limited | 227632171 | manufacture(69881-920, 69881-921) | |