MINTED LEAF ODORLESS HISTAMINE DHCL PAIN RELIEF AND HEMP EXTRACT- histamine dihydrochloride cream
MMG Consumer Brands, LLC
----------
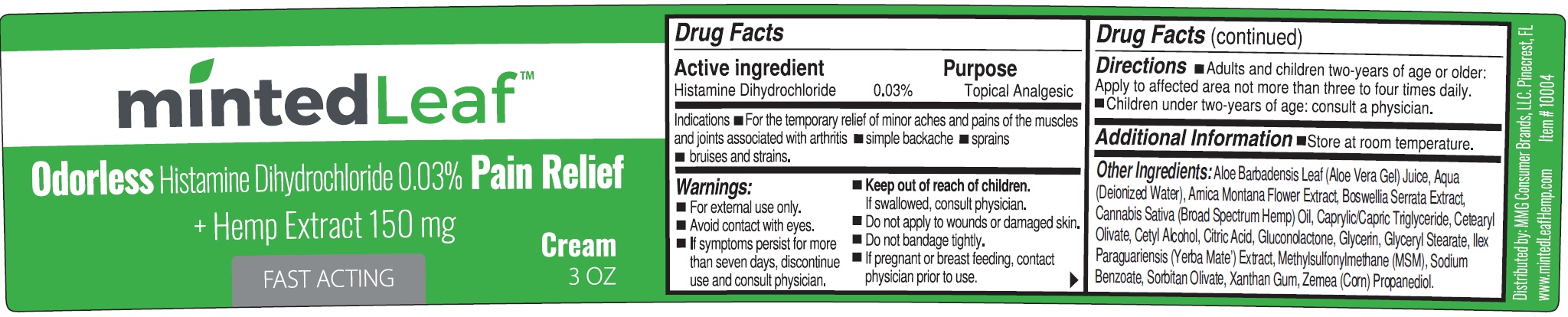
MINTED LEAF Odorless Histamine DHCl Pain Relief + Hemp Extract
Indications
- For the temporary relief of minor aches and pains of the muscles and joints associated with arthritis
- simple backache
- sprains
- bruises and strains.
Warnings:
- For external use only.
- Avoid contact with eyes.
- If symptoms persist for more than seven days, discontinue use and consult physician.
Directions
- Adults and children two-years of age or older: Apply to affected area not more than three to four times daily.
- Children under two-years of age: consult a physician.
Other Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cannabis Sativa (Broad Spectrum Hemp) Oil, Caprylic/Capric Triglyceride, Cetearyl Olivate, Cetyl Alcohol, Citric Acid, Gluconolactone, Glycerin, Glyceryl Stearate, llex Paraguariensis (Yerba Mate’) Extract, Methylsulfonylmethane (MSM), Sodium Benzoate, Sorbitan Olivate, Xanthan Gum, Zemea (Corn) Propanediol.
| MINTED LEAF ODORLESS HISTAMINE DHCL PAIN RELIEF AND HEMP EXTRACT
histamine dihydrochloride cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - MMG Consumer Brands, LLC (117036455) |
Revised: 10/2023
Document Id: 08ee0969-39e5-0aa9-e063-6394a90aa96f
Set id: 216f098a-3acf-4ae5-a9bc-267445777ff0
Version: 3
Effective Time: 20231030
MMG Consumer Brands, LLC