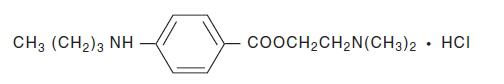Label: TETRACAINE HYDROCHLORIDE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 76413-127-15 - Packager: Central Texas Community Health Centers
- This is a repackaged label.
- Source NDC Code(s): 24208-920
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 20, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Tetracaine Hydrochloride is a sterile aqueous topical anesthetic ophthalmic solution. The active ingredient is represented by the chemical structure:
C15H24N2O2∙HCI
Mol. wt. 300.83Benzoic acid, 4-[butylamino]-, 2-[dimethylamino]ethyl ester, monohydrochloride.
EACH mL CONTAINS: ACTIVE: Tetracaine Hydrochloride 5 mg (0.5%); INACTIVES: Boric Acid, Potassium Chloride, Edetate Disodium and Purified Water. Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (3.7 - 6.0). PRESERVATIVE ADDED: Chlorobutanol 0.4%.
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- NOTE:
-
ADVERSE REACTIONS:
Transient symptoms (signs) such as stinging, burning and conjunctival redness may occur. A rare, severe, immediate allergic cornea reaction has been reported, characterized by acute diffuse epithelial keratitis with filament formation and/or sloughing of large areas of necrotic epithelium, diffuse stromal edema, descemetitis and iritis.
-
DOSAGE AND ADMINISTRATION:
For tonometry and other procedures of short duration, instill one or two drops just prior to evaluation. For minor surgical procedures such as foreign body or suture removal, administer one to two drops every five to ten minutes for one to three instillations. For prolonged anesthesia as in cataract extraction, instill one or two drops in the eye(s) every five to ten minutes for three to five doses.
- HOW SUPPLIED:
- STORAGE:
-
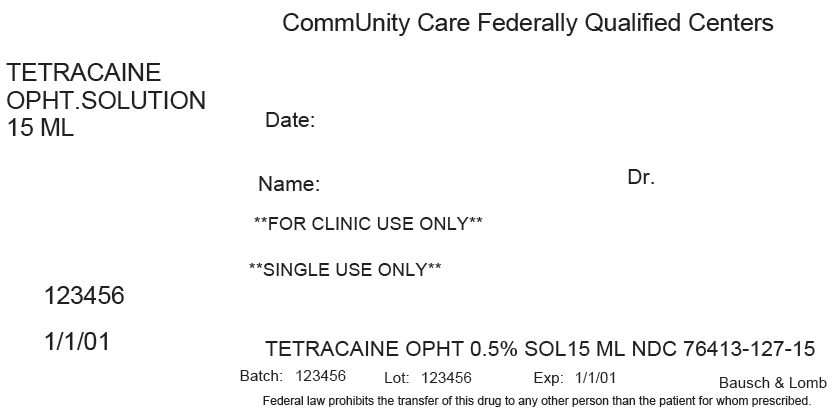
PRINCIPAL DISPLAY PANEL - 15 ML Bottle Label
CommUnity Care Federally Qualified Centers
TETRACAINE
OPHT.SOLUTION
15 MLDate:
Name:
Dr.**FOR CLINIC USE ONLY**
**SINGLE USE ONLY**
123456
1/1/01
TETRACAINE OPHT 0.5% SOL15 ML NDC 76413-127-15
Batch: 123456
Lot: 123456
Exp: 1/1/01
Bausch & Lomb
Federal law prohibits the transfer of this drug to any other person than the patient for whom prescribed.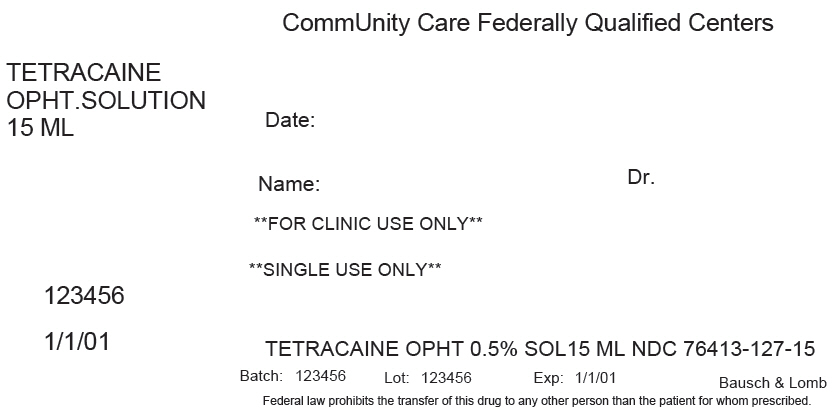
-
INGREDIENTS AND APPEARANCE
TETRACAINE HYDROCHLORIDE
tetracaine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76413-127(NDC:24208-920) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACAINE HYDROCHLORIDE (UNII: 5NF5D4OPCI) (TETRACAINE - UNII:0619F35CGV) TETRACAINE HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) POTASSIUM CHLORIDE (UNII: 660YQ98I10) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) CHLOROBUTANOL (UNII: HM4YQM8WRC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76413-127-15 1 in 1 CARTON 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 09/30/1990 Labeler - Central Texas Community Health Centers (079674019) Establishment Name Address ID/FEI Business Operations Central Texas Community Health Centers 079674019 REPACK(76413-127) , RELABEL(76413-127)